White Hybrid Light-Emitting Diodes: A Plausible Solution for Commercialization and Health Issues, by the University of Erlangen-Nuremberg (FAU)
One of the challenges in terms of commercialization of white LEDs is the need for cheap, inorganic phosphors. In this context, hybrid LEDs have recently emerged as a new approach based on downconverting organic coatings. The authors, Pedro B. Coto from the Institute for Theoretical Physics, Marlene Pröschel and Uwe Sonnewald from the Department of Biology, and Lukas Niklaus, Michael D. Weber and Rubén D. Costa from the Department of Chemistry and Pharmacy at the University of Erlangen-Nuremberg (FAU), describe this technology and cover recent breakthroughs and their prospects.
Nowadays, more than 20% of the total of domestic energy consumption is devoted to artificial lighting, but it is still dominated by incandescent light sources and compact fluorescent lamps. Generally speaking, although they have almost been fully developed, they are considered to be inefficient and environmentally unfriendly. Indeed, both the USA and the EU have stated that inorganic white light-emitting diodes (WLEDs) will drive the future of artificial lighting for both indoor and outdoor purposes [1]. This is based on their impressive theoretical efficiency limit of around 400 lm/W and the low-cost per lumen, which is falling a 10-fold every decade [2]. Indeed, recent market studies have estimated that over 14 billion Euros could be saved by replacing old illumination systems by WLEDs [1]. For instance, according to a McKinsey study, it is assumed that LEDs will account for 70% of the global lighting market in 2020, which corresponds to 101 billion Euros (Figure 1) [1a].
At the same time, due to economics of scale, production costs will fall to about 32% of 2014 values by 2020 [1]. These impressive expectations have been established for monochromatic LEDs, but not yet for WLEDs, due to the use of a top coating based on inorganic phosphors (IP) like Y3Al5O12; YAG:Ce3+ derivatives.
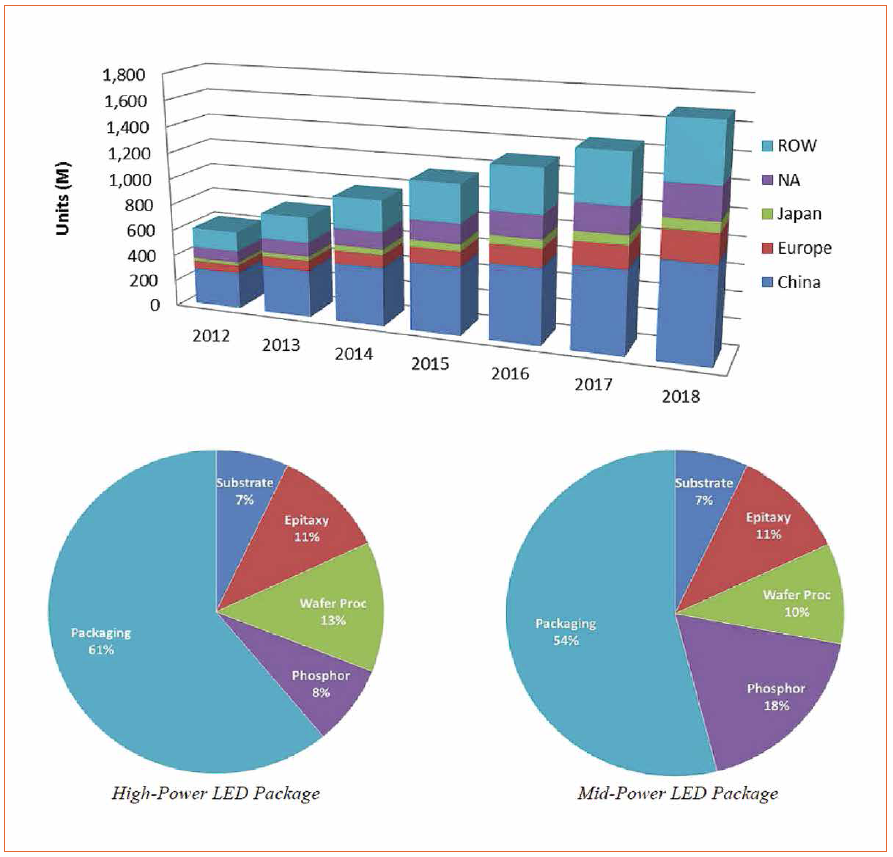
Figure 1: Top: Predictions of the growth of LED luminaire with respect to unit sales from 2012 to 2018 (Source: Smallwood, Strategies Unlimited, DOE SSL Manufacturing R&D Workshop, San Diego, 2012) Bottom: Cost distribution for high-and mid-power LED. (Source: LEDCOM model 1b)
About Concerns Regarding IP Coated LEDs
The main drawbacks of IP-based coating methods are:
• High production cost due to the need of a chemical vapor deposition method
• Intrinsic high cost of the raw materials, since they are very rare
• Lack of near-infrared and/or deep-red emitters, and
• Lack of cost efficient protocols to recycle the IPs [1, 3]
Thus, problems with the IP coatings will lead to a predicted increase of production costs of IPs by 5% until 2020. This is even more critical if we consider that today the IPs account for up to one fifth of the production costs of LEDs (Figure 1) [1, 3]. A switch to organic light-emitting diodes (OLEDs) for general illumination is still not possible, since OLEDs feature only half the efficiency and about a 50-times higher price compared to LEDs on a dollars per kilo-lumen-basis as well as still having issues with the lack of recycling protocols [4]. Thus, alternative IPs and/or approaches are sought by both the scientific and industrial communities, as this route does not meet the requirements of “Green Photonics” in terms of ecological sustainability, low energy consumption, and the use of low-cost and renewable materials [1, 3].
Besides the future market issues associated with the IPs, scientists and social institutions have started to evaluate the impact of WLEDs on human health [5]. Here, the question is: are LEDs as safe as old illumination systems? This addresses both visual and non-visual effects on human health caused by the strong brightness in concert with the light spectrum of WLEDs. The visual effects involve three major considerations, namely flickering, glare, and blue light hazard [5, 6]. Going from less to more dangerous concerns, it is expected that flickering will not be very important in the future, since most of the high quality LED sources feature rectifiers and filters that effectively reduce the light flickering to almost zero. However, this could be critical for people with a risk of suffering epileptic seizures - e.g., a few seconds exposition at 3-70 Hz and susceptibility to migraines and eye fatigue - e.g., a few hours exposition at 100-200 Hz. Glare is the second most dangerous factor if we consider high power outdoor lighting systems that can cause temporary and reversible loss of vision, damage to the eye tissues, etc. Still there are no international standards to evaluate this effect for single point sources. Finally, the so-called blue-light hazard is associated with the use of a high blue component ‑e.g. InGaN ‑in combination with yellowish orange emitting IPs that typically provides a cold white light spectrum. As a matter of fact, exposition of high-intensity blue light leads to retinal damage, such as the death of photoreceptors - i.e. necrosis and/or apoptosis - at wavelengths between 440-490 nm, and damage of retinal pigment epithelium by means of an autophagy process [6a-c]. Although our body has repair mechanisms, the photochemical damage is considered to be accumulative upon interrupted exposition times. Up to date, ANSI/IESNA RP27, CIE S009, and IEC 62471 are international standards to determine the blue-harmful light in terms of brightness, distance, number of sources, and exposition times [5, 6].
Here, the scale ranges from
• RG0 - i.e. no risk or unlimited exposition without hazard effect
• to RG1 - i.e. low risk at times ranging from 100-10000 s
• to RG2 - i.e. moderate risk at times of 0.25-100 s
• and finally, to RG3 - i.e. high risk at times lower than 0.25 s
Examples of RG2 LEDs involve indoor and outdoor lamps and luminaires with high brightness, such as automotive headlights, flash lights, frontal headlights, and some bright LED display panels. In general, many of the blue and cold white LEDs are considered RG1 or RG2, while white warm LEDs are all considered RG0. Importantly, these studies do not cover human cases like those dealing with the above-mentioned diseases, affecting children’s eyes that feature a lens that is more transparent to blue and UV radiation and a macula that does not contain enough protective yellow pigments, as well as old people, whose eyes have accumulated blue-sensitive lipofuscin derivatives with age. In addition, none of the above-mentioned studies consider the effects of lifelong cumulated exposures to blue light at both high- and low-dose exposure, as well as the effect of the UV irradiation or the lack of N-IR contribution compared to the sun light spectrum in, for instance, skin problems and/or vitamin D production.
Finally, the non-visual effects involve a change in brain chemistry induced by light exposition [5, 6d&e]. Although it is well-known that a high blue contribution in the light spectrum produces a decrease of the melatonin levels, affecting the circadian rhythms, mood, alertness, appetite, sleep quality, etc., a clear relationship with the illuminance level, the exposure duration, the timing of the exposure, and the light spectrum is not well established yet. However, it is well-known that warm white light - i.e., light with a dominant yellow, orange, and/or red contributions - is less effective in reducing melatonin than cold white light. It is important to keep in mind that, since the sun light spectrum changes over the course of a day, the human vision has become significantly sensitive to the changes in the solar spectrum - i.e. human eyes feature photopic (day), mesopic (sunset) and scotopic (dim-light) visions. As an example, our eyes are more sensitive to the blue component of the white LEDs (especially cool white LEDs) during the night.
White Hybrid LEDs as a Reasonable Alternative
Taking the above described concerns about commercialization and health seriously, it is obvious to state that although WLEDs will rule the future market [1, 3], there are clear needs to seek after new down-converting approaches to provide warm white LEDs that fulfill the green economic requirements [1, 3, 5]. In this context the potential of white hybrid light-emitting diodes (WHLEDs) sets in [7-10]. In detail, this technology aims to replacing IPs for organic down-converting materials. Here, the WHLED architecture consists of the same electronics and emitting chips as those developed for commercial UV- and blue-LEDs, but coating or replacing the packing system with a down-converting organic compound (Figure 2).
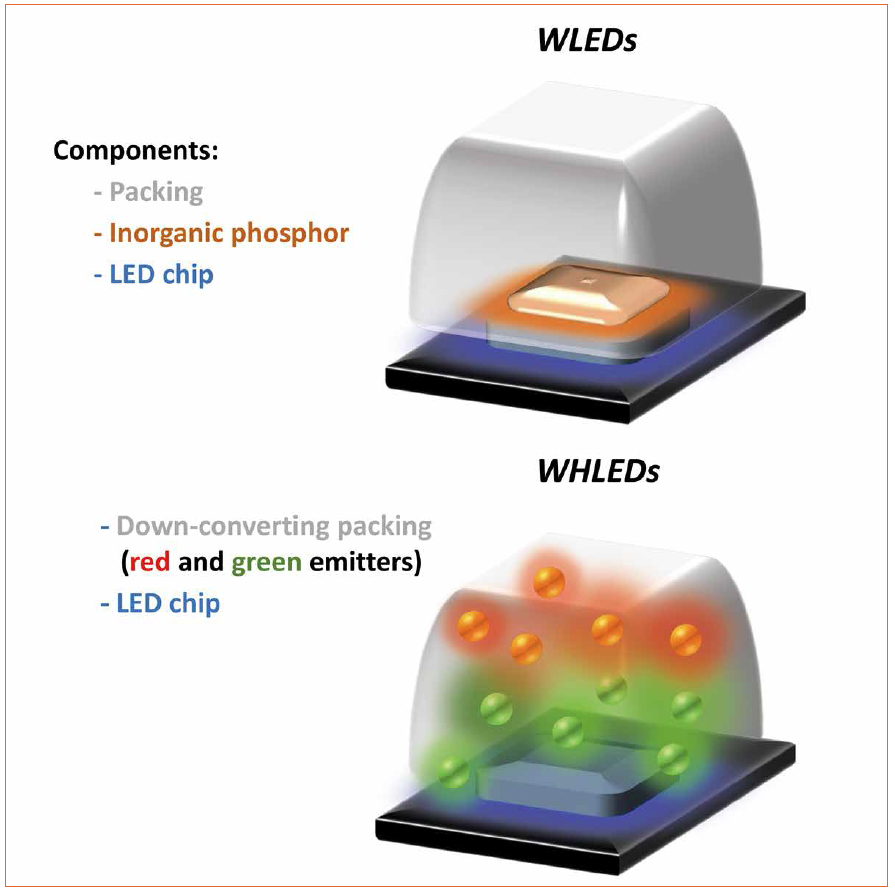
Figure 2: Representation of a commercial WLED (top) and WHLED (bottom) with organic down-converting packing coatings
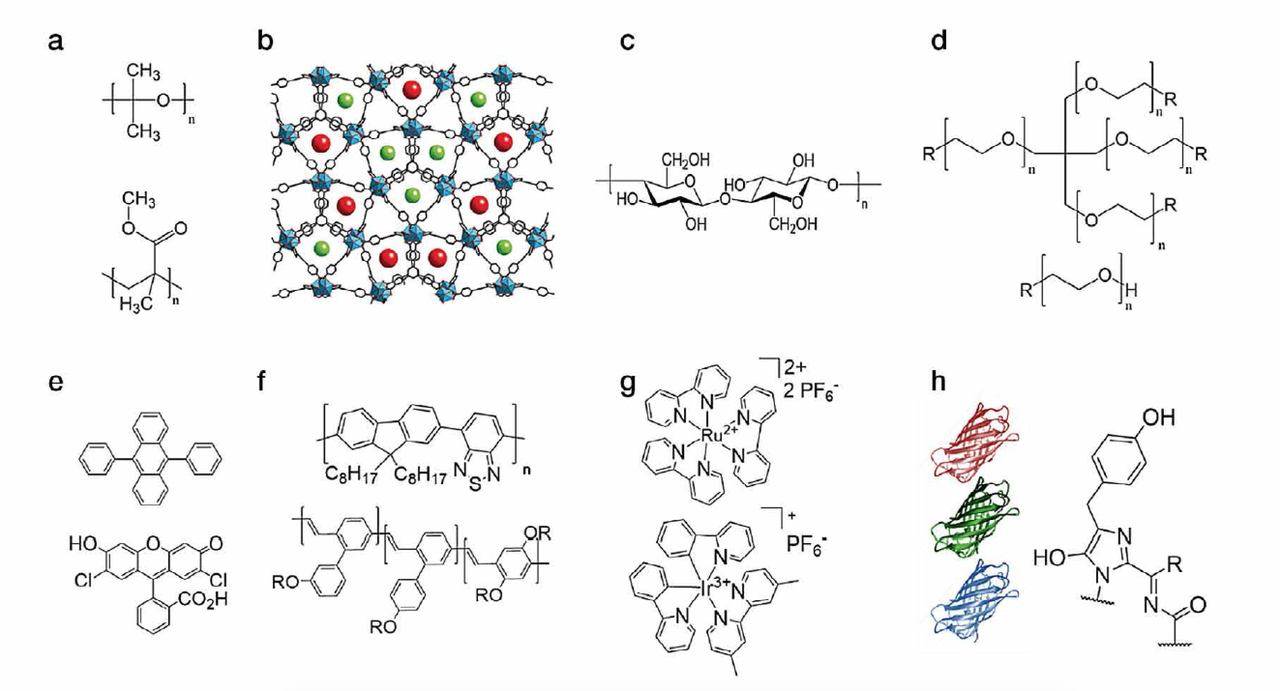
The latter is excited by the chip and the overall light spectrum of the WHLED involves both the emission of the LED (UV or blue) and the emission of the packing. Down-converting organic compounds consist of several families like small-molecules, polymers, quantum dots, coordination complexes, and most recently fluorescent proteins (Figure 3). Among them, small-molecules, polymers, and fluorescent proteins are the most interesting materials due to lack of toxicity, low-cost production/recycling, and ease of chemical design to cover all the visible and NIR regions towards mimicking the sun light spectrum.
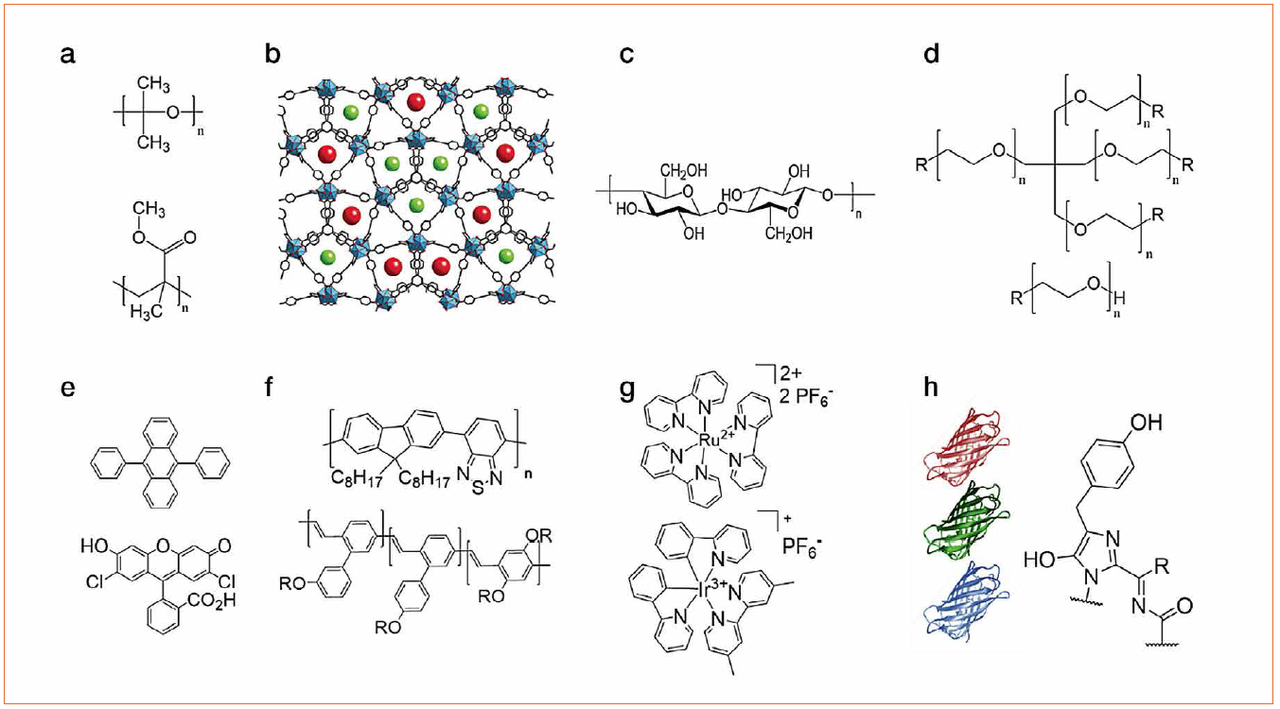
Figure 3: Top - chemical structures of the compounds used for developing matrices based on (a) thermal/UV curing polymers, (b) MOFs, (c) cellulose, and (d) rubber-like materials. Bottom - chemical structures of down-converting compounds, namely (e) small-molecules, (f) polymers, (g) coordination complexes and (h) fluorescent proteins
The state-of-the-art WHLEDs designed and tested under laboratory conditions show very promising results in terms of luminous efficiencies and color quality. For instance, WHLEDs have shown efficiency values of up to 60 lm/W with Commission International d’Eclairage (CIE) coordinates of 0.30 / 0.30, color rendering index (CRI) up to 90, and correlate color temperature (CCT) between 2500-6500 K. The only drawback is the moderate stability of around hundred hours under operation conditions. This is not totally related to the intrinsic photostability of the organic compounds, but also to their interaction with the components of the matrix chosen to encapsulate the organic compounds. Here, scientists have developed four different approaches [7-10]. In chronological order, several researchers have mixed fluorescent compounds like small-molecules, polymers, and quantum dots with matrices based on silicones and/or epoxies derivatives that require UV and/or thermal curing procedures to obtain a robust encapsulation [7a-d]. Here, only WHLEDs prepared with toxic quantum dots show interesting stabilities of at least several days [7b&d], while those with small-molecules and polymers feature only a few minutes or hours [7a&d]. As such, this approach has shown limitations in terms of degradation of the compounds upon preparation, phase separation issues, and lack of protocols to efficiently implement them into blue-LEDs. The most plausible approach is to previously encapsulate the organic compounds and then to implement them into the down-converting coating [7d]. The second approach involves the use of metal organic frameworks (MOFs) that show a porous morphology that allows to adsorb several mixtures of down-converting materials to cover the whole visible spectrum (Figure 3) [8]. Recently, this idea has been carried out with the use of three different non-toxic small-molecules that provide WHLEDs with CRI over 90 [8b]. This approach is easily applicable to any commercial LED and it is versatile with respect to the type of down-converting materials, but the stability in terms of luminous efficiency and color quality as well as the development of MOFs based on cheap an environmentally friendly metals still remain a challenge. Recently, several groups have developed a down-converting system based on a cellulose matrix and a mixture of blue, green, and red quantum dots [9]. The preparation of the composite is quick and facile to reproduce, but its compatibility with other materials and the stability of the WHLED needs to be assessed to elucidate its prospect.
Finally, we have recently developed a new encapsulation system that consists of mixing branched and linear polymers under vigorous stirring followed by the partial evaporation of the solvent under vacuum conditions [10]. This leads to a rubber-like material, in which any kind of down-converting material can be applied and easily recycled. Indeed, this matrix was developed to allow the use of fluorescent proteins as down-converting coating for WHLEDs. Hereafter, this new device will be referred to as Bio-WHLED. Fluorescent proteins are environmentally-friendly compounds and are easily produced by means of their expression in E. coli, which is a low-cost and up-scalable technique [11]. Despite these assets, the need of water-based buffer solutions and their low intrinsically stability under ambient conditions and high temperatures have hampered their use in the optoelectronic field. However, once they are embedded into the rubber-like matrix, the fluorescent proteins show stabilities of several months without affecting the features of the rubber-like encapsulation (Figure 4). Similar to the cellulose-based WHLEDs, different amounts and mixtures of the fluorescent proteins can be integrated into the rubber-like material, but we encountered that the best results are achieved by using a bottom-up energy transfer cascade coating (Figure 4). Here, the UV- and/or blue emission of the chip is absorbed by the protein coating that further emits light at a higher wavelength. More importantly, the ratio of down-conversion efficiency - i.e. the ratio between the maxima related to the down-converting coating and the chip - can be increased up to values of 400% by means of increasing the amount of proteins or the thickness of the coating (Figure 4). The excess of emission from the first coating can be used to excite a second coating that features emission at higher wavelengths. This procedure can be repeated as many times as required to cover up the whole visible spectrum (Figure 4). In addition, it can be extended to the NIR region, since the rubber-like encapsulation features transmittance values of around 95% and refractive index values superior to 1.8 and the cascade architecture circumvent energy loss issues due to undesired energy transfer process.
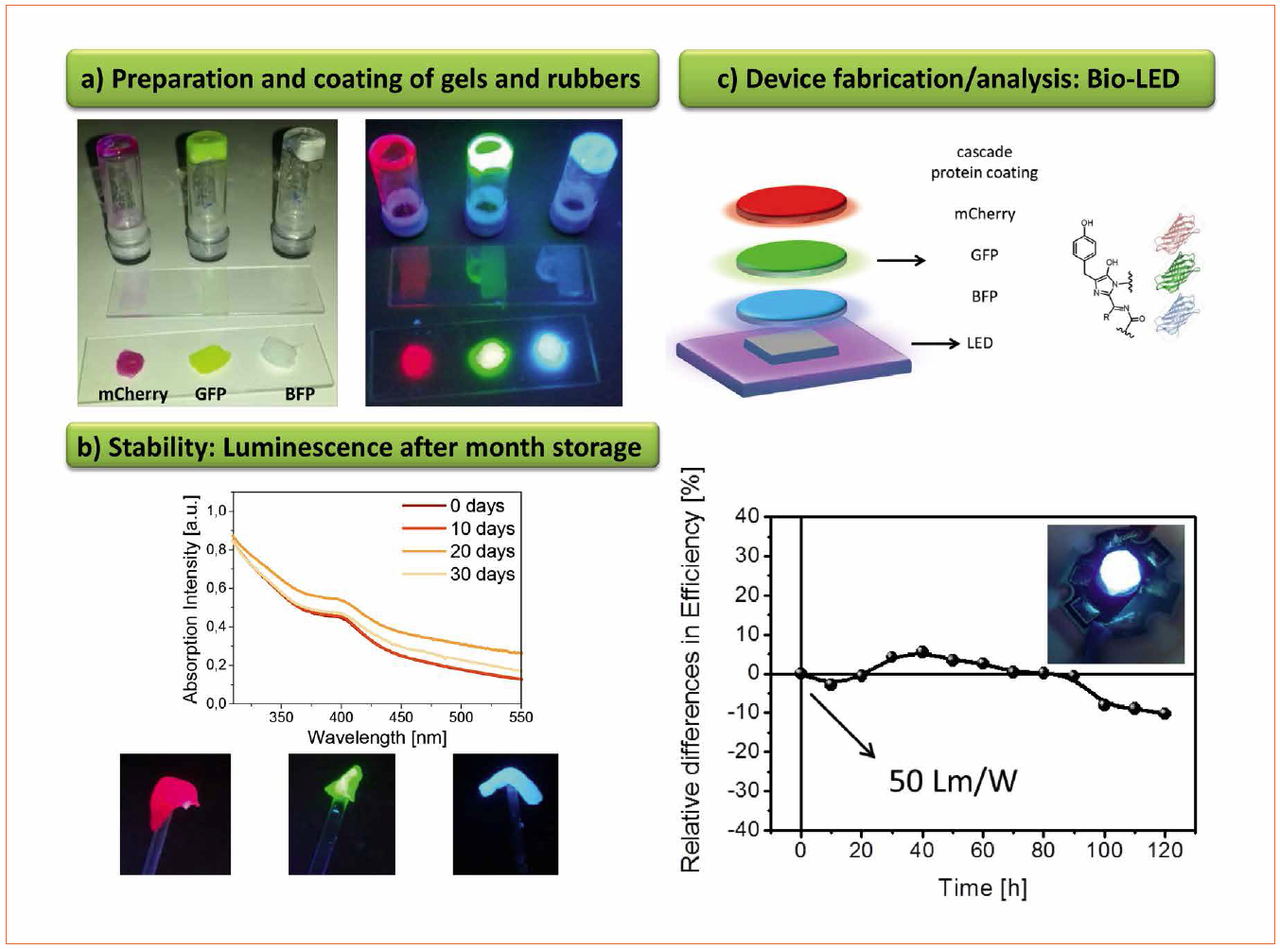
Figure 4: (a) Pictures of gels and rubber-like materials based on fluorescent proteins. (b) Top, absorption spectra over time suggest high stability under ambient storage. Bottom, pictures of the rubbers upon UV excitation. (c) The design of the first Bio-WHLEDs (top) and its performance under continuous working conditions (bottom)
In detail, the Bio-WHLEDs shown in figure 4 have been prepared by covering commercial UV- or blue- LED by a double- or triple-layer coatings - i.e. a blue LED (450 nm) and green (520 nm) / red (650 nm) or an UV LED (390 nm) and blue (480 nm) / green (520 nm) / red (650 nm) fluorescent proteins, respectively. These devices show a light spectrum that consists of the sum of the peaks of all the components of the LED - i.e. those from the chip and the down-converting packing, providing easy-to-tune light spectrum with, for instance, CIE coordinates of 0.32 / 0.33, CRI up to 80, and luminous efficiencies of 50 lm/W. More interestingly, the luminous efficiency and color quality stay constant over hundreds of hours with a loss of less than 10% of its initial values (Figure 4).
In view of the aforementioned, WHLEDs can be considered as an attractive approach in terms of replacing the expensive and rare IPs, resulting in a strong reduction of the total prize of the LED, and efficiently converting the blue emission of the chip to the more necessary low-energy component for developing warm white emitting LEDs that are not dangerous to human health.
WHLEDs can easily use filters and attenuators to avoid the flickering and the glare issues. Though the only roadblock seems to be stability towards the commercial implementation of the technology. This is even more critical if we consider the development of high power LED arrays, in which high temperatures are reached. To this end, two major considerations need to be addressed in the near future.
The packing system must feature:
• An easy-to-fabricate protocol without using thermal/UV curing procedures
• Low cost raw materials and recycling protocols
• High transmittance, refractive index, and mechanical robustness
The down-converting compounds must be:
• Easy and cheap to produce
• Nontoxic
• Easy to tune in terms of high absorption extinction coefficients and fluorescent quantum yields
• Thermo- and photo-stable under ambient conditions
Conclusions
Taking these considerations into account, the Bio-WHLEDs bear great potential for future breakthroughs. However, more efforts need to be devoted to circumvent the loss of color quality that is related to denaturation of the proteins and to enhance the thermal stability of both the matrix and the proteins. Currently, we are designing more thermo- and photo-stable fluorescent proteins and polymers to further enhance the performance of the Bio-WHLEDs featuring warm white emissions and even more relevant to mimic the sun light spectrum.
References
[1] a) McKinsey & Company, Lighting the way - Perspectives on the global lighting market / second edition, 2012; b) US Department of Energy, Life-Cycle Assessment of Energy and Environmental Impacts of LED Lighting Products, 2012; Top Sector HTSM - Innovation Contract, Lighting the future, 2013; US Department of Energy, Manufacturing Roadmap Solid-State Lighting Research and Development, 2014.
[2] Haitz, R. et al. Phys. Status Solidi A, 2011, 17, 208.
[3] Federation of National Manufacturers Association for Luminaires and Electrotechnical Components for Luminaires in the European Union & European Lamp Companies Federation (CELMA & ELC), The European Lighting Industry’s Considerations Regarding the need for an EU Green Paper On Solid State Lighting, 2011.
[4] a) Dehen, W. CEO of OSRAM in ZVEI: AMPERE - Das Magazin der Elektroindustrie (http://www.zvei.org/Publikationen/ZVEI-AMPERE-1-2014.pdf) 2014; b) Volz, D. et al. Green Chem., 2015, 17, 1988.
[5] a) The Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR of the European Commission, Health Effects of Artificial Light, 2012. http://ec.europa.eu/health/ scientific_committees/opinions_layman/artificial-light/en/index. htm#1; b) Martinsons, C. et al. International Energy Agency 4E Solid State Lighting, Solid State Lighting Annex - Potential Health Issues of Solid State Lighting Final Report 2014.
[6] a) Jaadane, I. et al. Free Radic Biol Med. 2015, 84, 373; b) Shang, Y. M. et al Environ Health Perspect. 2014, 122, 269; c) Marquioni-Ramella, M. D. et al. Photochem. Photobiol. Sci., 2015, 14, 1560; d) Bonmati-Carrion, M. A. et al. Int. J. Mol. Sci. 2014, 15, 23448; e) Madrid-Navarro, J. et al. Current Pharm. Design, 2015, 21, 3453.
[7] a) Huyal, I. O. et al. J. Mater. Chem. 2008, 18, 3568; b) Jang, E.-P. et al. Nanotechnology 2013, 24, 045607; c) Yoo, H. et al. Nanoscale, 2015, 7, 12860; d) Findlay, N. J. et al. Adv. Mater. 2014, 26, 7290.
[8] a) Sun, C.-Y. et al. Nat. Commun. 2013, 4, 2717; b) Tetsuka, H. et al. J. Mater. Chem. C 2015, 3, 3536; c) Cui, Y. et al. Adv. Funct. Mater. 2015, 25, 4796.
[9] a) Zhou, D. et al. ACS Appl. Mater. Interfaces 2015, 7, 15830; b) Tetsuka, H. et al. J. Mater. Chem. C 2015, 3, 3536.
[10] a) Weber, M. D. et al. Adv. Mater. 2015, 27, 5493; b) Weber, M. D. et al. Rubber-like Material for the Immobilization of Proteins and its Use in Lighting, Diagnosis and Biocatalysis EP-1674, 15173026.4 (pending)
[11] a) Shcherbakova, D. M. et al. Current Opin. Chem. Biol. 2014, 20, 60; b) Chudakov, D. M. et al. Physiol. Rev. 2010, 90, 1103.