Circadian-Friendly Light Emitters: From CCT-Tuning to Blue-Free Technology
Common levels of blue radiation are sufficient to disrupt the circadian cycle, calling for blue-depleted emitters in the evening. Most current solutions employ low-CCT and suffer from poor light quality. Aurelien David, chief scientist at Soraa explains the melanopic lumen and its possible sources of inaccuracy related to the uncertainty in assessing the circadian action spectrum. In addition, he also discusses the concept of a blue-free emitter with minimal melanopic lumen at very low CCT and how such a spectrum providing a good light quality can be optimized.
The last two decades of research have revealed a significant effect of blue radiation on our circadian cycle, calling for suitable light emitters. The basics of these physiological effects of blue light are first reviewed. Metrics to measure these effects are introduced, and variations in the estimated circadian action spectrum are illustrated. Strategies to influence circadian entrainment are discussed, including CCT-tuning and more advanced spectral engineering. Finally, the specific challenge of offering low-entrainment light emitters is highlighted; a solution based on the novel blue-free technology is presented, and shown to break the trade-off between light quality and circadian entrainment plaguing conventional LED sources, with applications in general lighting and displays.
Light and Sleep – Scientific Foundations
Light has long been known to influence the circadian cycle through non-visual channels. In the early 2000's, significant progress in understanding was achieved when the specific vector for this stimulus was discovered [1,2]: besides the four visual cells, the human retina comprises a non-visual cell type, the intrinsically photosensitive retinal ganglion cell (ipRGC).
The study of the circadian effects of light is a still-evolving field. However, a few results are well accepted. The ipRGCs are maximally-sensitive to blue-cyan light, with a peak around 460-490 nm. ipRGC stimulation causes direct non-visual physiological signals – inducing the suppression of melatonin and other hormones – and has a direct influence on our circadian cycle [3,4]. This stimulation is governed by the total dose of stimulating radiation reaching the ipRGCs (i.e. the amount of radiation, weighed by the ipRGC sensitivity) [5]. From an evolution standpoint, this sensitivity is understandable: morning daylight is bright and rich in blue radiation, providing a natural synchronization signal.
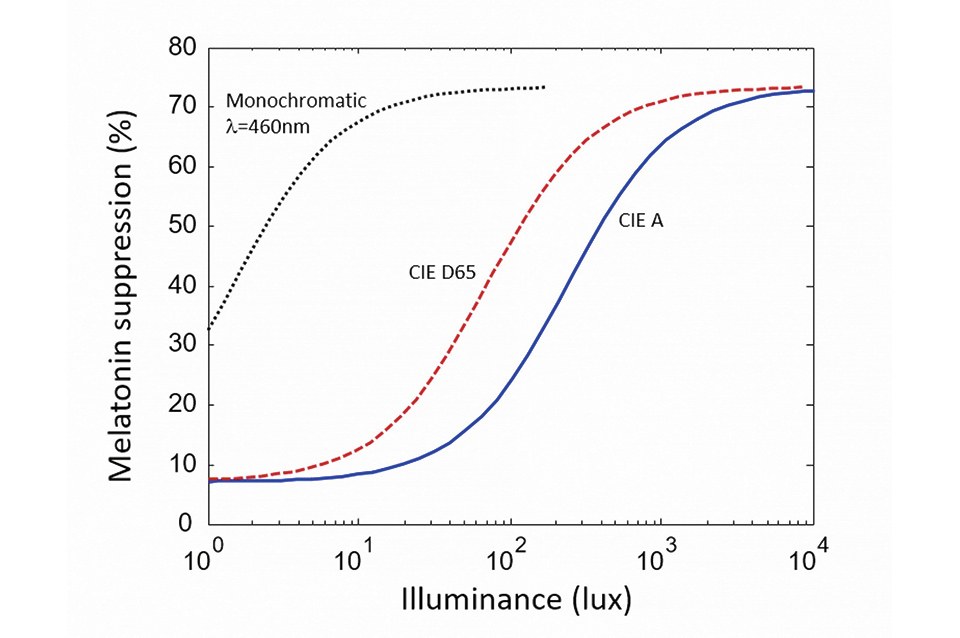
Figure 1 shows the melatonin suppression (after two hours of light exposure) versus illuminance, for various light sources. The dashed curve is after the experimental data of [6] and shows the response to monochromatic blue light. The other two curves are derived from this data and show the response for two white-light reference illuminants: respectively CIE A (2,800 K) and CID D65 (6,500K). In each case the curve has a so-called sigmoidal shape: it saturates for very low and high retinal illuminance levels. Note the logarithmic scale of the x-axis indicating that, in the sensitivity region, a large-enough change in illuminance (typically a factor of two to ten) is required to significantly affect melatonin suppression
The effect of blue radiation on our sleep cycle is a form of circadian entrainment. Entrainment is often evaluated experimentally by measuring the suppression of melatonin in saliva after exposure to light. Melatonin suppression only has an indirect relationship to sleep (contrary to common belief, melatonin does not cause sleep per se, and instead indicates exposure to obscurity). However, because melatonin is much easier to measure than quality of sleep in experiments, it constitutes a convenient proxy for discussions of light and circadian effects.
Crucially, melatonin suppression exhibits a non-linear response to the total dose of stimulating light, as illustrated in (Figure 1). Foundational research on the circadian impact of light was first conducted with monochromatic blue radiation [6], for which the relationship between dose and melatonin suppression was directly measured. From these measurements, equivalent curves can be derived for white light sources by considering the amount of blue radiation in their spectra. As seen in
Importantly, it has been shown that regular amounts of indoor artificial light are also sufficient to cause circadian stimulation [7,8].
Given this medical understanding, the lighting industry has been trying for the last few years to develop products which respect or influence our circadian cycle – an approach often described under the umbrella term "human-centric lighting". Two essential aspirations of human-centric lighting are to offer high circadian stimulation in the morning (to help synchronize our internal clocks) and limited stimulation in the hours before bedtime (to avoid sleep disruption). This has proven to be a challenging task, in part because the underlying scientific understanding is only partial, making it difficult to ensure that products indeed provide the benefits they claim.
Quantifying Circadian Entrainment – Methods and Uncertainties
A central challenge is to translate the general concepts described above into simple quantities, enabling engineers to develop a light source. The main approach retained today is to compute an effective amount of circadian stimulation by weighing the SPD of a light source with a so-called circadian action spectrum (an estimate of the wavelength-sensitivity of our circadian system). The result of this calculation is sometimes called the melanopic lux: a quantity derived in analogy with the well-known lux, but where the photopic sensitivity curve V() is replaced by the circadian action spectrum. Given two sources of same illuminance, the melanopic lux compares their relative potential for entrainment.
The concept of melanopic lux implies significant simplifications. For instance, it ignores the dynamic aspects of circadian response – including the fact that the impact of a light source depends strongly on the history of prior light exposure during the day [5]. Further, it assumes that only the ipRGCs influence the circadian system, whereas it is known that visual receptors also have an influence. These simplifications, although significant, appear necessary given our state of knowledge to enable engineering applications.
Another source of uncertainty comes from the estimation of the circadian action spectrum itself, which isn't an easy task. It took decades of careful work for color scientists to determine the spectral sensitivity of the corneal cones, which dictate our color vision – and similar research regarding the ipRGCs is ongoing. The current estimates all agree that the spectral sensitivity is maximal around 460-490nm, but they differ in the shape of their short- and long-wavelength tails.
Figure 2 shows two action spectra retained by standard-setting entities: the International Lighting Commission (CIE) and the German Institute of Standards (DIN), illustrating such differences in sensitivity at short and long wavelength. Yet other estimates in literature suggest the action spectrum may be narrower.
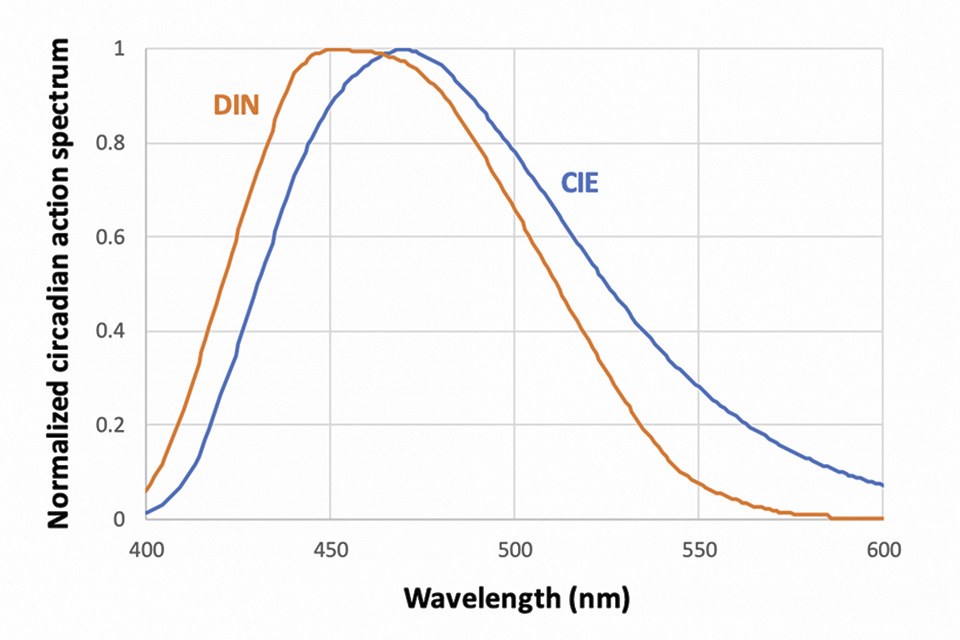
Figure 2: Examples of circadian action spectra, proposed by the CIE and the DIN
The CIE action spectrum in particular has garnered some consideration. It is the basis for the melanopic lux calculation in the software toolbox of Ref. [9] – which will be used in this Article. It forms the basis for human-centric specification in the recent WELL building standard
Spectral Engineering for Circadian Entrainment
These calculations enable the engineering of a spectral power distribution (SPD) for increased or decreased circadian stimulation, an opportunity which has garnered much attention in the lighting community. In the following, various approaches are reviewed.
CCT tuning
Given the maximal sensitivity of the action spectrum to blue radiation, one naturally expects that emitters of higher correlated color temperature (CCT), which emit more short-wavelength radiation, should cause more entrainment. This is indeed the case, as illustrated on (Figure 3a), which shows the fraction of blue light and the melanopic lumen for blackbody radiators of various temperatures: the melanopic lux roughly increases between 2,500 K and 5,000 K.
Figure 3a shows the melanopic lux for a series of blackbody radiator emitters of varying CCTs, each with an illuminance of 100 lux. The higher amount of blue radiation at higher CCT translates into a higher melanopic lux. Figure 3 b shows a comparison of two LED SPDs with a same CCT of 5,000 K. Deep blue curve: conventional white LED with a blue-pump LED having a peak wavelength 440 nm (CRI Ra=80 / R9 = 0, melanopic lux = 75). Light blue curve: LED with blue-cyan pump LED and modified phosphors (CRI Ra = 70 / R9 = 0, melanopic lux = 105)
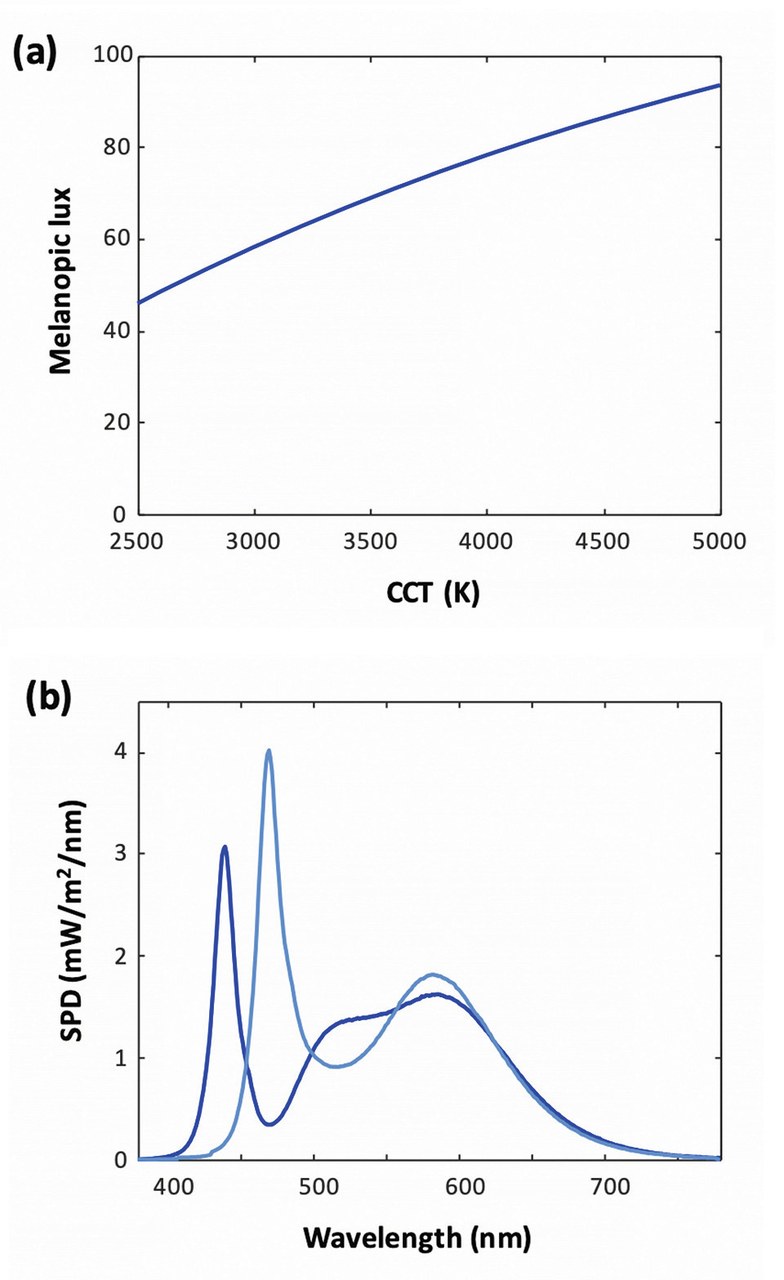
Figures 3a&b: Melanopic lux for a series of blackbody radiator emitters of varying CCTs (a) and SPDs for two LEDs at CCT 5000 K (b)
It should be kept in mind, however, that CCT tuning must be considered together with illuminance levels to have a meaningful effect on circadian entrainment, because circadian response saturates at low and high light levels. This is seen in (Figure 1). Comparing the melatonin suppression of an A-lamp (2,800 K) and a cool white emitter (6,500 K), the difference is sizeable at moderate illuminance levels (around 100 lux), but small or even negligible at low and high illuminance (below 10 lux or above 1,000 lux).
Therefore, CCT-tuning can indeed be impactful, provided it is combined with an appropriate level of light – i.e. a few hundred lux, where our circadian system is most sensitive. Fortunately, such lighting levels are in fact typical of indoor lighting. The effect of CCT-tuning in indoors lighting has been documented in studies [10].
Blue-enriched spectra for increased stimulation
Beyond the CCT trend discussed above, lighting manufacturers are seeking to engineer SPDs in order to further influence entrainment for a given CCT. For LED emitters, this can be achieved by selecting proper wavelengths for the pump LEDs and phosphors, to manipulate the detailed shape of the SPD.
A well-known example is that of "blue-enriched" SPDs. Given the objective to maximize entrainment in the morning, the first step is to use a high CCT as just discussed, but the lighting designer may decide that there is a maximum acceptable value – say 5,000K. Given this CCT, the SPD can be optimized to increase melanopic lux. (Figure 3(b)) shows an example, where a conventional blue-pump LED (=440nm) is replaced with a blue-cyan LED (=470nm), resulting in a 40% relative increase in melanopic lumens. This comes at a cost on color rendition however, since in this example the CRI drops from 80 to 70 – these specific values aren't fundamental, yet they illustrate the existence of a trade-off between circadian entrainment and color rendition.
Blue-enriched SPDs can of course be use in more complex lighting systems, where they are combined with a tunable CCT and a tunable illuminance, in order to deliver maximal stimulation at the proper time of the day. Some research supports the validity of such a "daylight-simulating" approach [11].
Blue-depleted spectra for decreased entrainment
The converse problem – that of minimizing stimulation in the evening, especially in the two hours before bedtime – poses more significant conceptual challenges. Fundamentally, this is because nearly-all LED technology today is predicated on the use of blue pump LEDs with peak wavelengths most commonly in the range 440-460 nm, making it impossible to completely remove blue radiation.
Figures 4 show blue-depleted SPDs for reduced entrainment. All emitters have the same illuminance (100 lux). Figure 4ademonstrates the relationship between CCT and melanopic lux for blackbody emitters (line),and various LEDs (dots).Blue-pumped LEDs (whether they are conventional or "sleep-friendly")closely follow the behavior of Blackbody emitters. In contrast,blue-free LEDs break this trade-off and offer a conventional CCT(here, 2,700 K) together with a low melanopic lux. Figures 4b show representative SPDs of a conventional (blue-pumped) white LED, a blue-pumped "sleep" LED,and a violet-pumped blue-free LED.
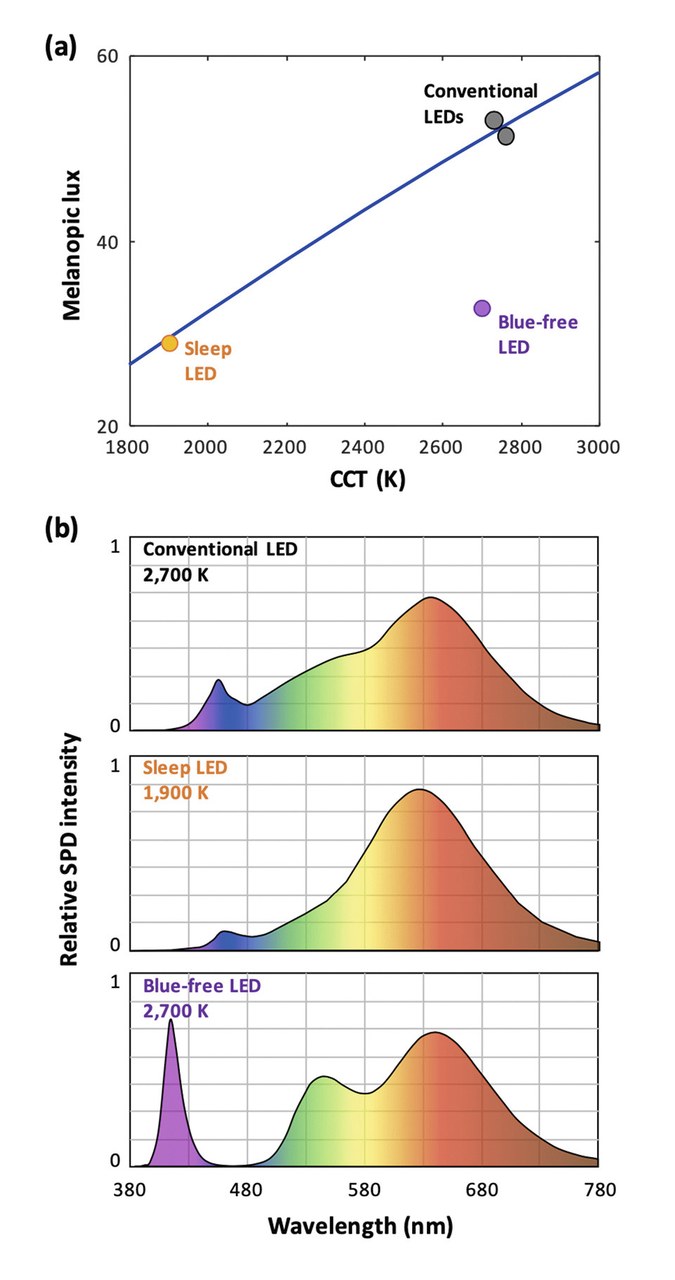
Figures 4a&b: Blue-depleted SPDs for reduced entrainment. CCT vs. melanopic lux comparison (a) and SPDs (b) of a conventional (blue pumped) white LED, a blue-pumped "sleep" LED, and a violet pumped blue-free LED
Conventional white LEDs, with blue-pump LEDs peaking around 450nm, display a melanopic lux very close to this curve, with small variations depending on the specific phosphor used – showing that in this case, standard spectral engineering isn't a sufficient tool to reduce the circadian lux.
In past years, the lighting industry has tried to address this issue by offering blue-depleted LEDs. However, this can only be obtained by reducing the CCT of the emitter. This is because blue is an essential part of the SPD of a white LED, required to color-balance it for a given CCT: when forcing a lower blue peak, the CCT must also be reduced.
Therefore, the resulting "sleep-friendly" LEDs merely show a melanopic lux compatible with their reduced CCT, nearly identical to a low-CCT blackbody radiator. In practice, such low CCTs (around 2,000 K) are practically equivalent to candle light and display a pronounced yellow cast, which is unacceptable in most lighting applications – let alone to light up a whole home for extended hours before bedtime.
In summary, conventional blue-based LEDs suffer from a fundamental trade-off whereby low circadian stimulation is tied to low CCT.
Blue-free emitters: a fundamental solution for low circadian entrainment
In past years, efficient white lighting based on violet pump LEDs has emerged as a viable alternative to the conventional blue-pump LED scheme. This was in large part enabled by the development of ultra-high efficiency violet LEDs. So far, violet-pumped white LEDs have mostly shown an advantage in applications where high quality of light is sought – in this case, they are combined with three phosphors (including a blue phosphor) to produce a full-spectrum with wide wavelength coverage.
However, violet-pump LEDs also offer a unique advantage for circadian-friendly lighting. Indeed, by omitting the blue phosphor and selecting the other phosphors appropriately, they enable white light where blue radiation is virtually absent, as shown on (Figure 4(b)) [12]. Despite this, a desired CCT (in this case, standard warm-white at 2,700 K) can be achieved by retaining a sufficient amount of violet in the SPD. As seen in (Figure 4a), this source nonetheless offers a melanopic lux similar to a 2,000 K conventional source.
Therefore, such blue-free SPDs break the trade-off between blue radiation and CCT described earlier, enabling low circadian entrainment without compromising the color of the light itself. Perhaps more surprisingly, the SPD if (Figure 4b) also displays excellent color metrics (CRI Ra=80, R9=90) – making it acceptable for widespread use in residential and hospitality settings.
From Lighting to Displays?
Concerns about blue light and circadian disruption are not limited to architectural lighting – electronic displays also cause the same effects. Conventional displays, with a CCT of about 6,500 K and a large blue peak, naturally provide high circadian entrainment, and there is little desire to further increase it. Rather, there is interest in reducing entrainment. Indeed, studies have confirmed that the use of displays at night could have a disruptive impact on sleep [13].
The previous discussion, while aimed at lighting applications, is also directly relevant for displays. Today, the approach retained in the industry is to reduce the display's CCT at night. This function is offered by the well-known software utility f.lux, and has been integrated in smartphones (e.g. Apple's Nightshift mode). However, just as in lighting, reducing CCT can only be taken so far before the color of the screen appears too warm. A low-point of 3,500 K is often proposed in this approach.
Here again, blue-free technology offers a path by enabling reduced circadian entrainment (on the order of 50%), even for high-CCT. Importantly, with proper engineering of color filters, this can be obtained while retaining the display's color quality: for instance, a DCI-P3 color gamut can be obtained and, contrary to common expectations, a saturated-blue primary can be achieved despite the absence of blue radiation in the SPD.
Conclusions
Given the significant effects of light on the circadian cycle, there is little doubt that human-centric lighting will proliferate in coming years. Ever-increasing scientific understanding will enable more relevant solutions. As it stands, the main challenge for the lighting industry is to offer dynamic lighting systems that can modulate circadian entrainment – at a minimum by tuning CCT, and beyond this with advanced spectral engineering. For years, the lighting industry has been able to offer a good solution to one half of this problem, by combining high illuminance and a blue-enriched SPD. With the advent of blue-free technology, enabled by highly-efficient violet pump LEDs, it is now possible to tackle the other half of this challenge and provide architectural-grade lighting with reduced circadian entrainment.
References:
[1] D. M. Berson, "Phototransduction by Retinal Ganglion Cells That Set
the Circadian Clock," Science (80-. )., vol. 295, no. 5557,
pp. 1070–1073, 2002
[2] S. Hattar, H. W. Liao, M. Takao, D. M. Berson, and K. W. Yau,
"Melanopsin-containing retinal ganglion cells: architecture, projections,
and intrinsic photosensitivity.," Science, vol. 295, no. 5557,
pp. 1065–70, Feb. 2002
[3] C. Dibner, U. Schibler, and U. Albrecht, "The Mammalian Circadian
Timing System: Organization and Coordination of Central and
Peripheral Clocks," Annu. Rev. Physiol., vol. 72, no. 1, pp. 517–549,
Mar. 2010
[4] T. A. LeGates, D. C. Fernandez, and S. Hattar, "Light as a central
modulator of circadian rhythms, sleep and affect," Nat. Rev. Neurosci.,
vol. 15, no. 7, pp. 443–454, Jul. 2014
[5] J. J. Gooley, S. M. W. Rajaratnam, G. C. Brainard, R. E. Kronauer,
C. A. Czeisler, and S. W. Lockley, "Spectral Responses of the Human
Circadian System Depend on the Irradiance and Duration of Exposure
to Light," Sci. Transl. Med., vol. 2, no. 31, p. 31ra33-31ra33, May 2010
[6] G. C. Brainard and J. P. Hanifin, "Action Spectrum for Melatonin
Suppression: Evidence for a Novel Circadian Photoreceptor in the
Human Eye," in Biologic Effects of Light 2001, Boston, MA:
Springer US, 2002, pp. 463–474
[7] J. J. Gooley, K. Chamberlain, K. A. Smith, S. B. S. Khalsa,
S. M. W. Rajaratnam, E. Van Reen, J. M. Zeitzer, C. A. Czeisler,
and S. W. Lockley, "Exposure to Room Light before Bedtime
Suppresses Melatonin Onset and Shortens Melatonin Duration
in Humans," J. Clin. Endocrinol. Metab., vol. 96, no. 3, pp. E463–E472,
Mar. 2011
[8] R. G. Stevens and Y. Zhu, "Electric light, particularly at night,
disrupts human circadian rhythmicity: is that a problem?," Philos. Trans.
R. Soc. B Biol. Sci., vol. 370, no. 1667, pp. 20140120–20140120,
Mar. 2015
[9] R. J. Lucas, S. N. Peirson, D. M. Berson, T. M. Brown, H. M. Cooper,
C. A. Czeisler, M. G. Figueiro, P. D. Gamlin, S. W. Lockley,
J. B. O'Hagan, L. L. A. Price, I. Provencio, D. J. Skene,
and G. C. Brainard, "Measuring and using light in the melanopsin age,"
Trends Neurosci., vol. 37, no. 1, pp. 1–9, Apr. 2014
[10] S. A. Rahman, M. A. St. Hilaire, and S. W. Lockley, "The effects of
spectral tuning of evening ambient light on melatonin suppression,
alertness and sleep," Physiol. Behav., vol. 177, pp. 221–229,
Aug. 2017
[11] V. Gabel, M. Maire, C. F. Reichert, S. L. Chellappa, C. Schmidt,
V. Hommes, A. U. Viola, and C. Cajochen, "Effects of Artificial Dawn
and Morning Blue Light on Daytime Cognitive Performance, Well-being,
Cortisol and Melatonin Levels," Chronobiol. Int., vol. 30, no. 8,
pp. 988–997, Oct. 2013
[12] M. R. Krames and A. David, "US9410664B2 - Circadian friendly LED
light source," US9410664B2, 09-Aug-2016
[13] A.-M. Chang, D. Aeschbach, J. F. Duffy, and C. A. Czeisler,
"Evening use of light-emitting eReaders negatively affects sleep,
circadian timing, and next-morning alertness.," Proc. Natl. Acad. Sci. U.
S. A., vol. 112, no. 4, pp. 1232–7, Jan. 2015