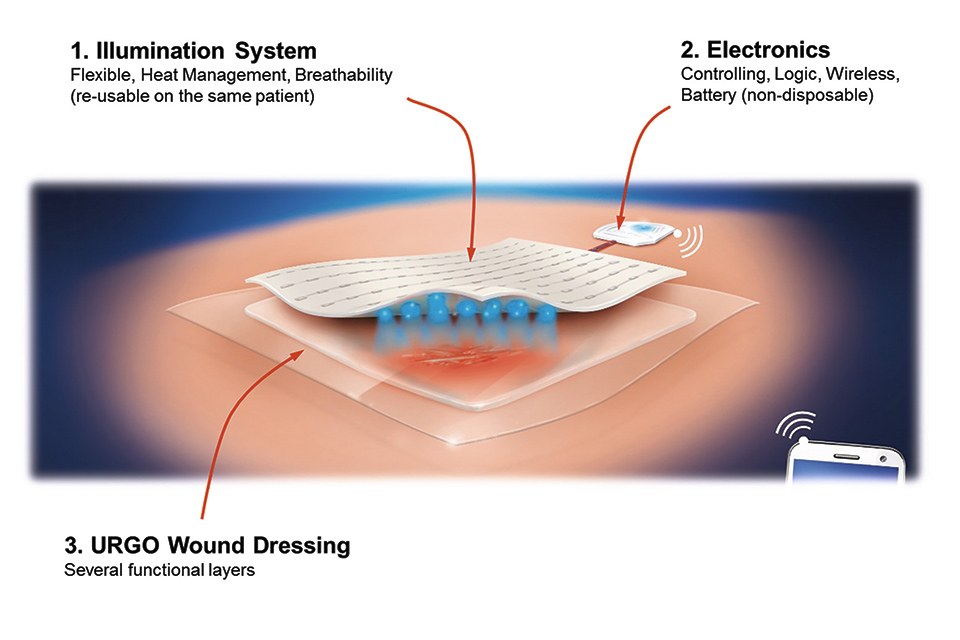
Flex LED Based Smart Light System for Healing of Chronic Wounds
LpR 71 Article - page 48: Chronic wounds are notoriously challenging to treat, because they do not follow the typical healing process or time-frame. The resulting burden is significant, affecting over 40 million patients. Blue light is known for its anti-microbial and anti-inflammatory effects in the initial stages of the healing process. David Kallweit, researcher at CSEM, reports about the joint development of a chronic wound treatment device with their MEDILIGHT partners, URGO RID, University of Heidelberg, SignalGenerix, Microsemi, Technical University of Berlin, and Amires. He furthermore shows how it works and discusses future prospects.
The Basics of Wound Treatment with Light
The key to the efficient wound treatment with the developed device lies in the mechanically flexible implementation of a very thin, energy-efficient and homogenous illumination system in combination with an elaborated lighting scheme which determines the applied optical power density and the duration of the treatment subject to the current phase of therapy, e.g. bacterial infection, cleansing, granulation of deeper skin cells (fibroblasts), and epidermization and closing of the wound (keratinocyte cells).
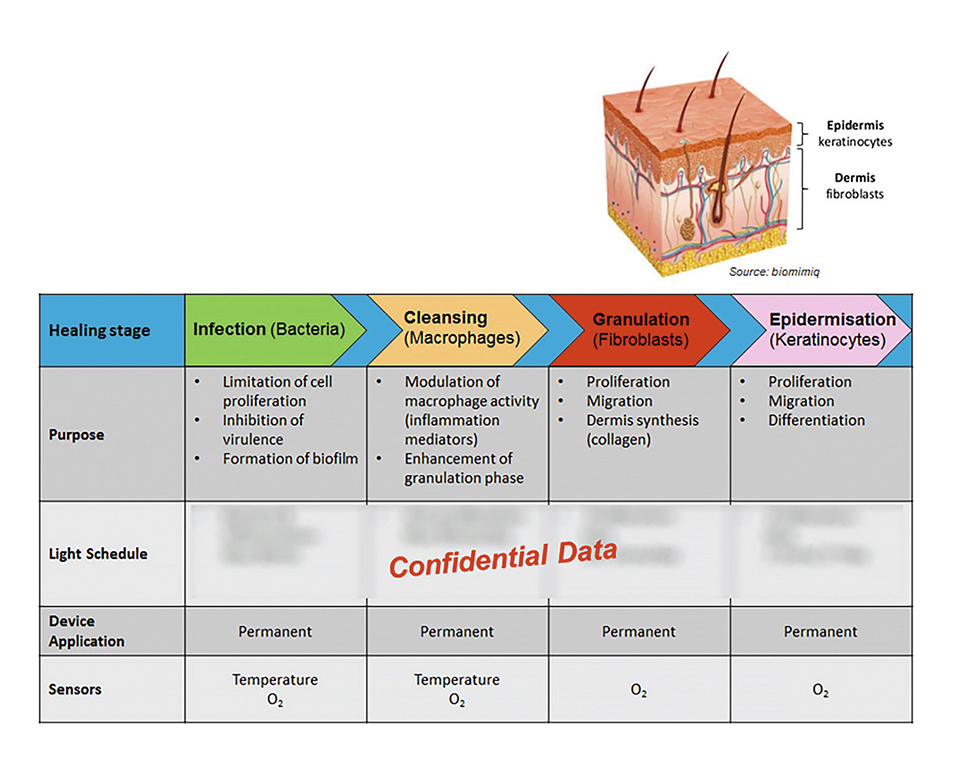
Figure 2: Different wound healing stages
The system also includes sensors to spatially measure the blood oxygenation below the wound, as well as sensors to monitor the temperature. The latter is especially used to ensure that a defined maximum temperature level is not exceeded during the light treatment, thus avoiding the formation of an algesia with the patient. In order to reduce the generated heat to a minimum, a set of measures have been implemented like highly efficient LEDs and diffusor films, light harvesting and back reflection of misdirected light, very-low loss wound dressings, and structures for heat conduction and ventilation.
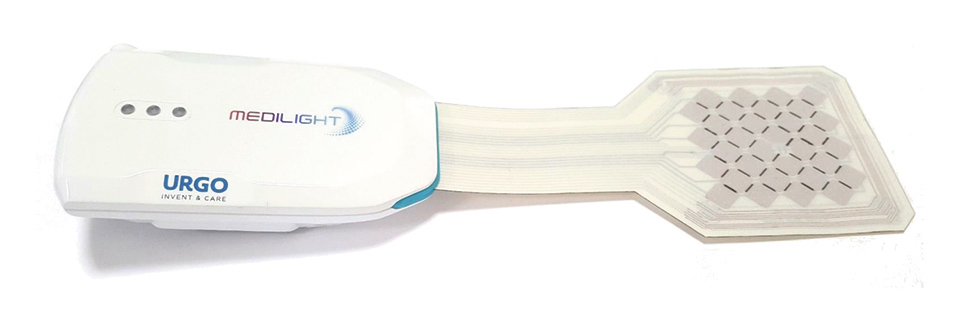
Figure 3: Fully functional prototype
The whole system can be controlled via Bluetooth and a sophisticated mobile app. It not only allows to apply a certain illumination, but also to program personalized sets of light treatments which individually define point in time, duration, and light intensity, and can also monitor the temperature and blood oxygenation and thus provide a personal treatment history.
The Lighting System
The lighting system consists of two main parts: the controlling electronics and software and the mechanically flexible LED foil with light and heat management and the wound dressing optimized for minimal optical loss.
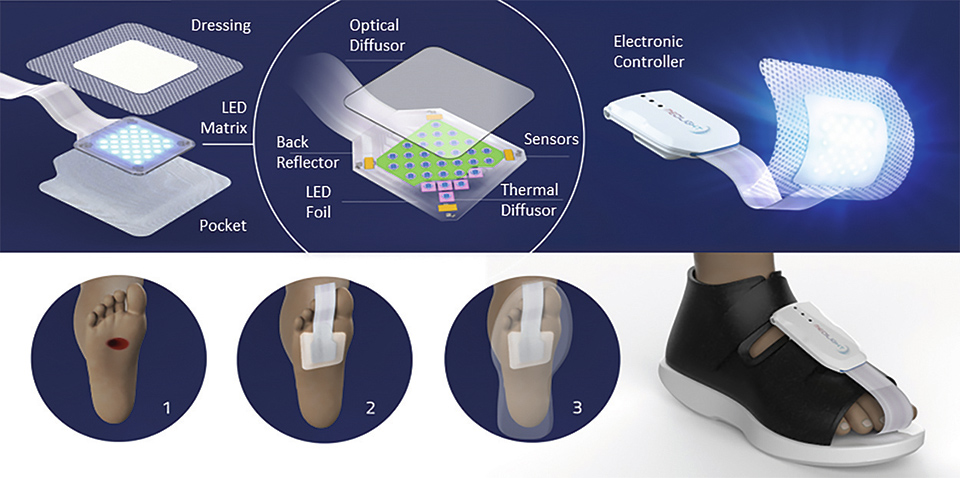
Figure 4: Illustration of the system and its application
In order to find the best compromise between high illumination homogeneity, but also minimal power loss different diffusor design were built and put under test. The diffusors were placed first in direct contact and then at increasing distances from the LED foil. At each position the intensity distribution was measured and a measure for the homogeneity was calculated. The result is shown in figure 5.
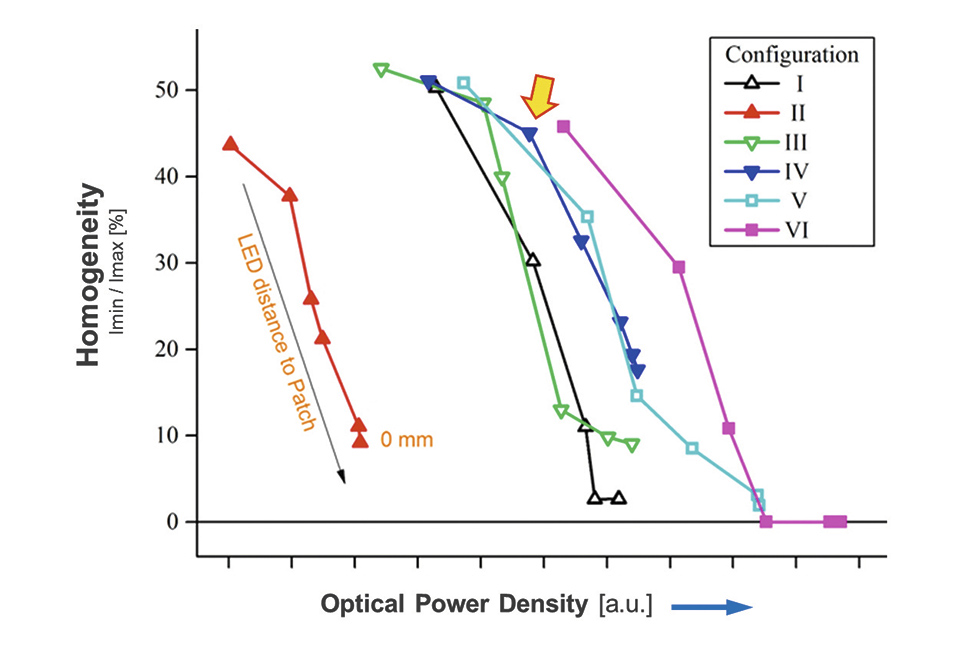
Figure 5: Summary of the different diffusors being tested
As one can see, and would have expected, the illumination homogeneity increases with increasing distance to the LEDs. On the other hand, the transmitted optical power is also reduced and thus the efficacy of the illumination system is reduced. Finally diffusors "IV" was selected, as it provided quite good homogeneity together with an acceptable efficacy of about 75% (specifications of diffusor and used light intensity is confidential due to possible patent application).
The light for the device is generated by a flex LED foil which is combined with a thin combination of diffusor foils. In order to improve the overall energy efficiency the flex LED foil is equipped with another back reflector foil, which collects the light that is accidently reflected at the diffusor layer and redirects it towards the wound. Finally, two optical designs were found where he diffusor can be either placed at a certain distance in order to gain a higher illumination homogeneity, or it can even be placed in direct contact to the LED foil, yet providing enough homogeneity of illumination for the biological effects of killing of bacteria and proliferation of skin cells to be effective. Figure 6 shows the two possible optical setups (diffusor with spacer and in direct contact). The corresponding measured illumination profiles are shown on top. The illuminated area shown is 4x4 cm.
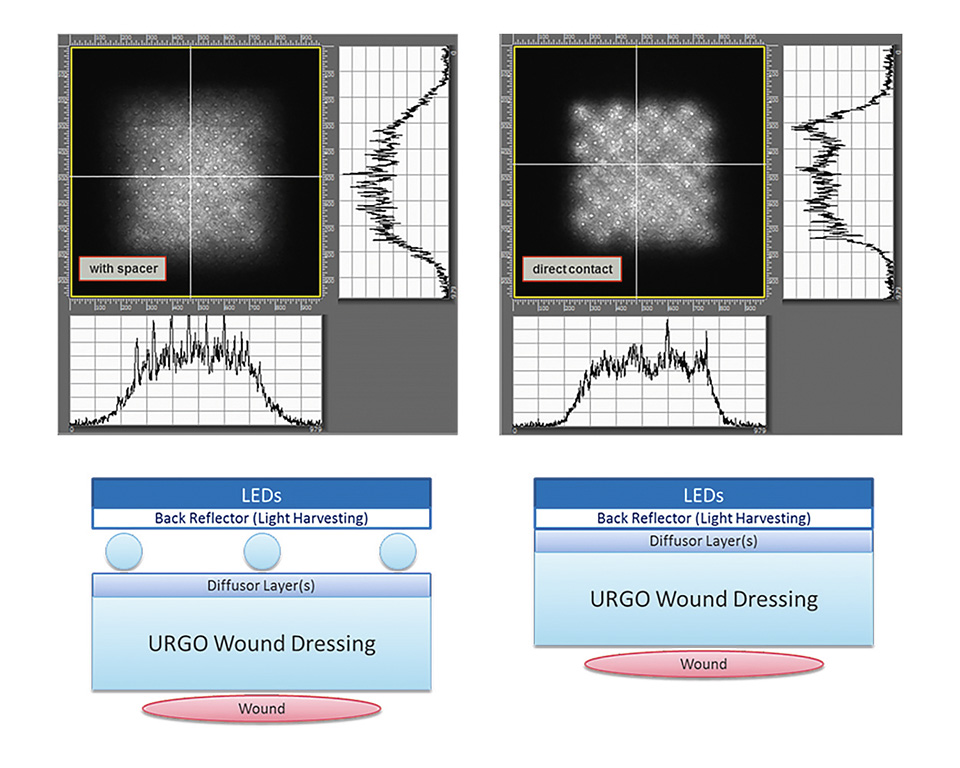
Figure 6: Illustration of the two optical setups and the corresponding illumination profiles
In order to reduce the heat generated by the LEDs the flex LED foil, some air vents and copper heat sinks have been implemented in the flex LED foil.
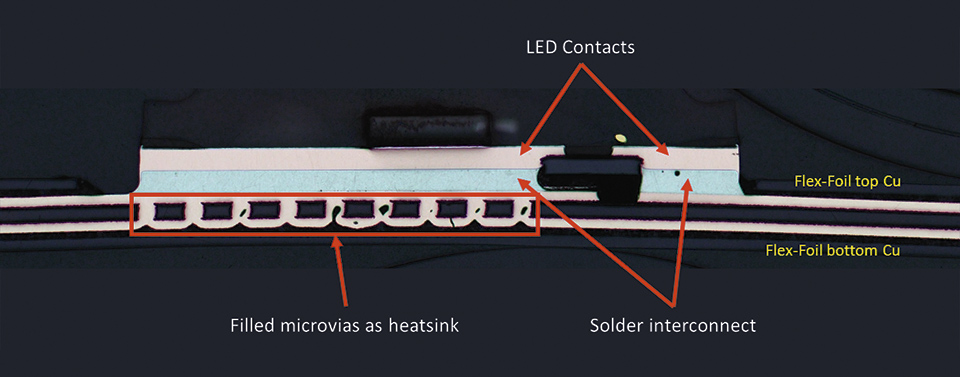
Figure 7: Cross section through mounted LED on flex foil
For safety reasons (to avoid overheating and damage to the patient) 5 sensors continuously monitor the temperature. One is located in the center of the illumination area and the other at the four corners. If the temperature threshold of (currently) 40°C threshold is reached the illumination is stopped until, through passive cooling (blood circulation, copper heat sinks, and air vents) the lower threshold of (currently), 37°C is reached again. Then the illumination is started again.
In order to reduce the heat generated by the LEDs they are also driven in pulsed mode. By correspondingly increasing the total duration of the illumination, it is made sure that the total required energy budget is delivered to the wound, which is needed to allow the desired biological effect to kick in.
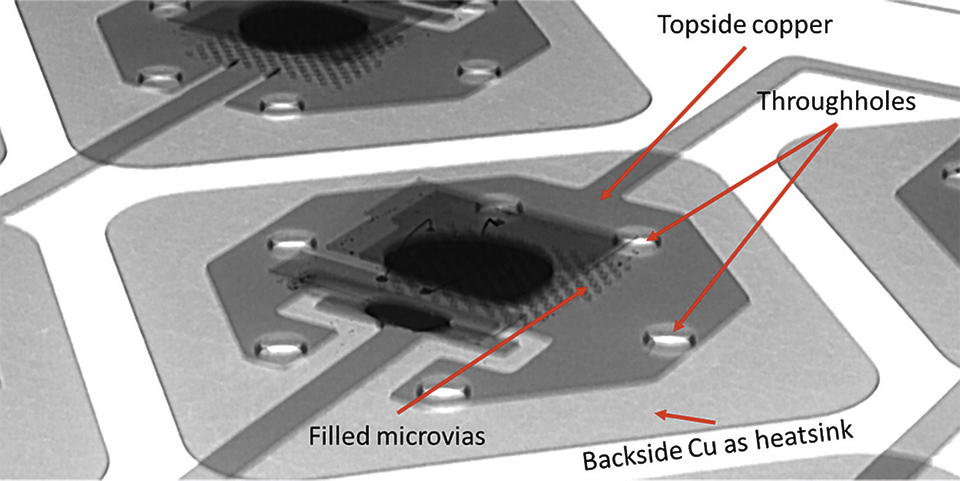
Figure 8: X-Ray image of a mounted LED
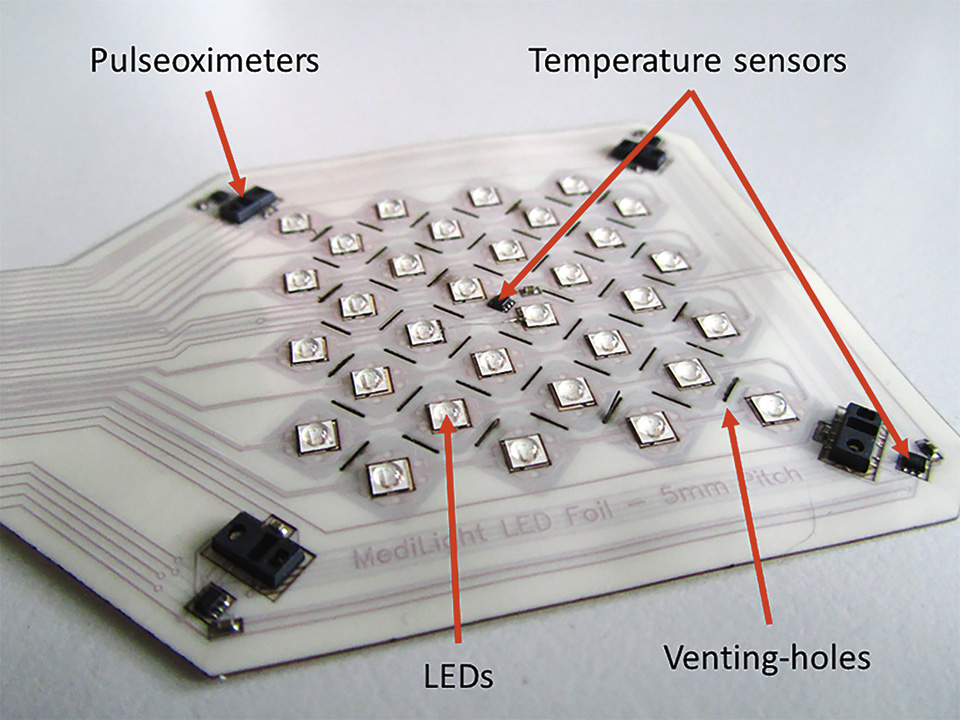
Figure 9: Flex-foil with mounted components
Finally the flex LED foil is also equipped with a sensor system, which allows for the measurement of local blood oxygenation as well as a mapping of the oxygenation of the muscle (across the wound area). Therefore, 4 pairs of red and infrared LEDs and photodiodes are placed in each corner of the wound dressing. By measuring the emitted light within sensor 1 only, one measures the local blood oxygenation. By measuring the light emitted from the opposing LEDs one can also measure the oxygenation of the muscle below the wound area.
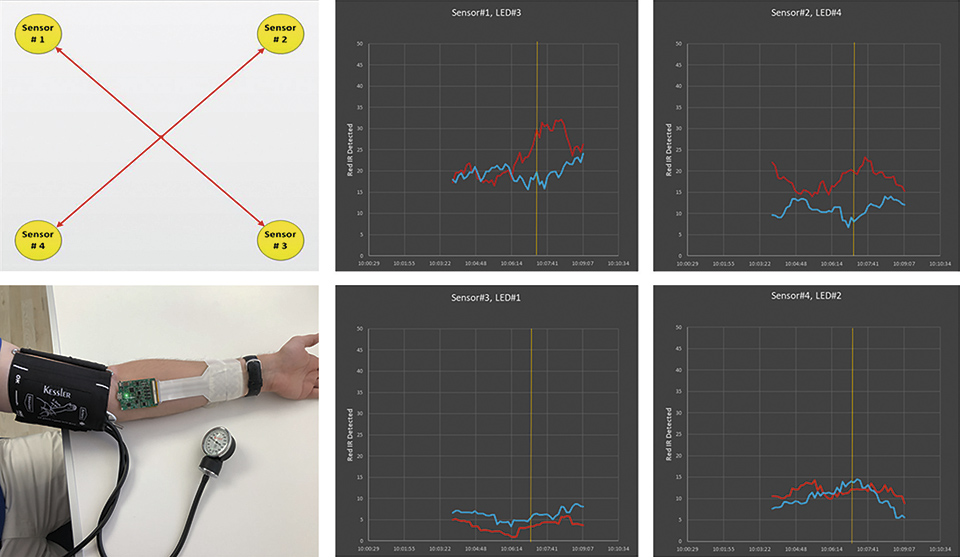
Figure 10: Implemeted sensor for measuring the blood oxygenation at the wound. On the left the mapping across the wound area is shown. On the right the measurement signals for the received red and infrared light that is received from the opposing LEDs is shown
The illumination system is controlled by a small box which can be easily attached to a shoe. Via a mobile app which communicated with the electronic box it is possible to control and program the illumination system. The app also allows to administer a list of devices and patients, which can be individually selected. Thus, subject to the current wound healing status of the respective patient, the doctor can chose and setup the corresponding treatment (illumination) procedure (infection, cleansing, granulation, and epidermization) individually.
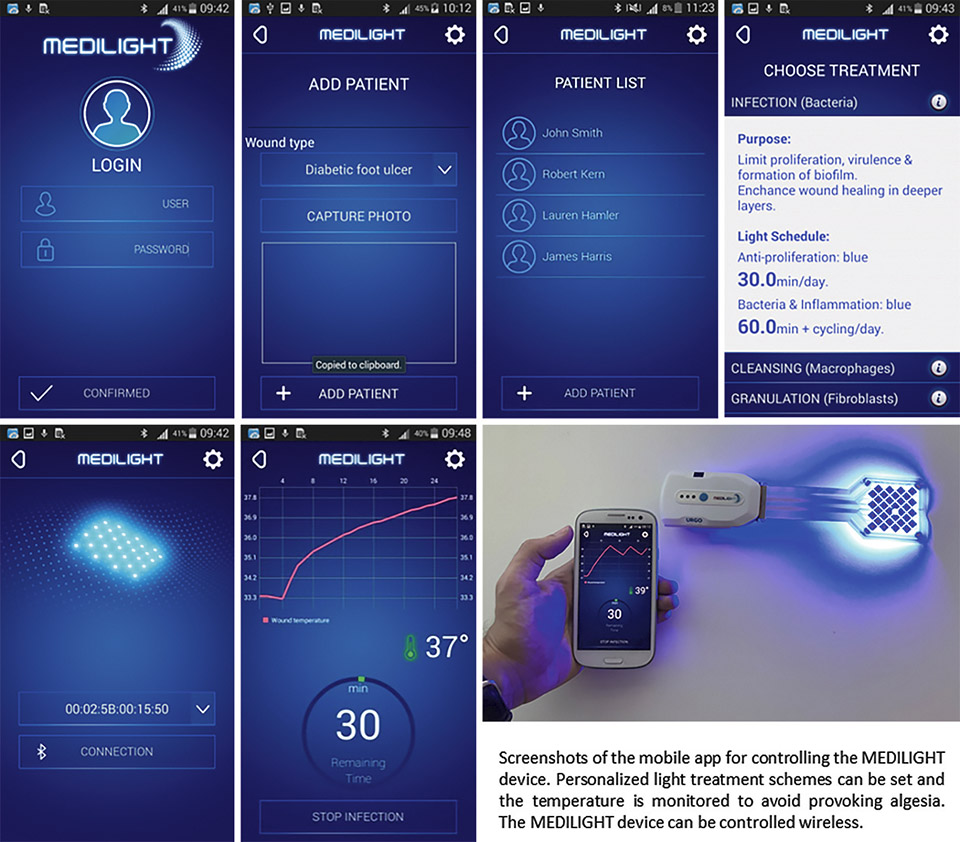
Figure 11: Screenshots of the developed mobile app which allows to control the illumination device
Besides setting the light treatment the app also allows monitoring the temperature situation and history as well as the measurement of the blood oxygen – which takes place when then wound illumination is switched off. All the light treatments and measurements can be organized and pre-programmed based on an individual schedule. The collected data is stored and can be read and transferred to a secured data server / cloud service.
Biological Effects
According to the literature, light therapy can be applied for a variety of medical conditions, especially for skin disorders along with tissue regeneration. Non-healing, chronic wounds are characterized by recurrent inflammatory processes, impaired cell function and molecular deficiencies within their microenvironment [1]. The anti-microbial and anti-proliferative effects of blue light are well known and explicitly described in literature [2,3]. In order to improve the wound healing process, MEDILIGHT proposed the use of blue light in early wound healing stages in order to inhibit or even abandon bacterial growth. In addition, it is important to prevent an overshooting epidermization by keratinocytes at the wound surface leading to a premature closure of the trauma. Contrary, red light is supposed to stimulate cell proliferation, migration and differentiation [4], which is decisive in later healing stages leading to a closure starting from the wound bed.
Light schedules using different wavelengths and dosages were at first tested and identified using skin cell types such as keratinocytes and fibroblasts as well as various bacterial strains like Staphylococcus aureus and Pseudomonas aeruginosa, which are the most prominent strains in chronic wounds [5]. The impact of the chosen irradiation schedule was further tested using healthy and diabetic rats to prove the transfer from in vitro to in vivo.
In vitro studies using infra-/red light have not shown the desired effects in keratinocytes and fibroblasts, which is not in agreement with most of the studies published (data not shown). However, lower doses with a defined energy of blue light were found to stimulate cell activity and proliferation. In contrast, higher doses, with a maximum at an irradiation time of 30 min of blue light, slow down cell metabolism (Figure 1) as well as proliferation confirming the anti-proliferative effect without leading to apoptosis [6,7].
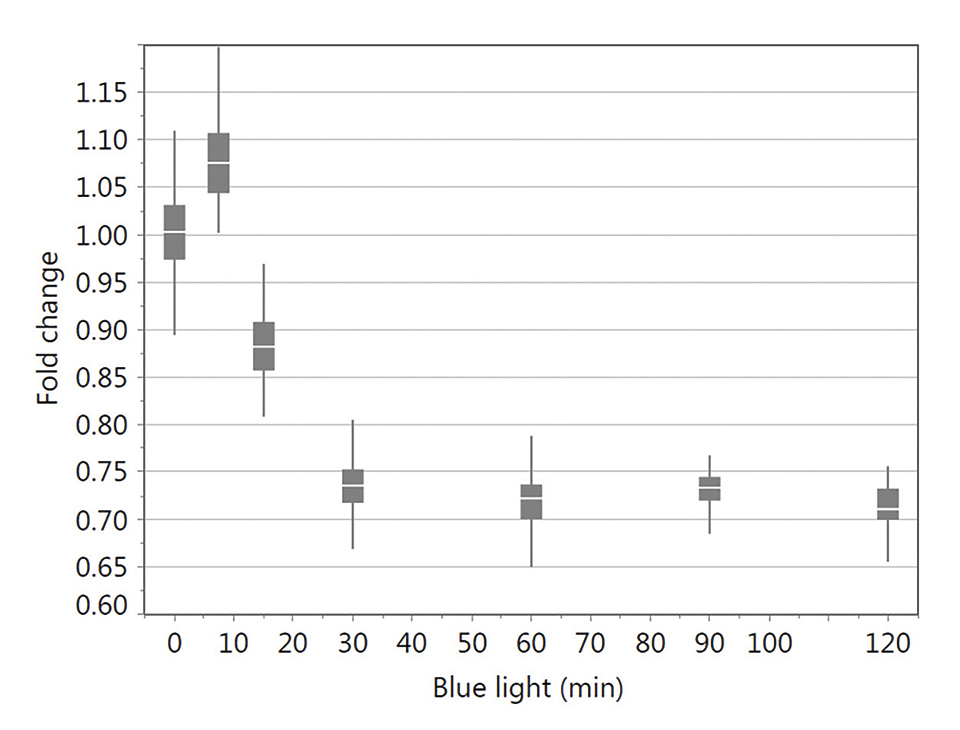
Figure 12: XTT test results for keratinocytes irradiated with different blue light exposure times (0, 7.5, 15, 30, 60, 90 and 120 min) tested 24 h following light stimulation. Data were normalized using the no light control. The box-and-whisker plots represent the distribution of XTT data values. These are ranked into quartiles, which divide the data set into a box of four equal groups; the band inside the box reflects the median. The whiskers extend from the ends of the box to the outermost data point that falls within the distances calculated as follows: 3rd quartile+1.5*(interquartile range) and 1st quartile-1.5*(interquartile range) (modified from6)
Directly following irradiation, levels of reactive oxygen species (ROS) got highly increased leading to the activation of several signaling pathways and downstream processes identifying the aryl hydrocarbon receptor as a possible target for photobiomodulation with blue light [7].
In vitro tests studying the effect of blue light on bacterial strains, revealed promising results with respect to colony count and size analyzed with AutoCellSeg, which is a software analyzing colony counts automatically by adaptive image segmentation and giving room for post-editing [8] (Figure 2).
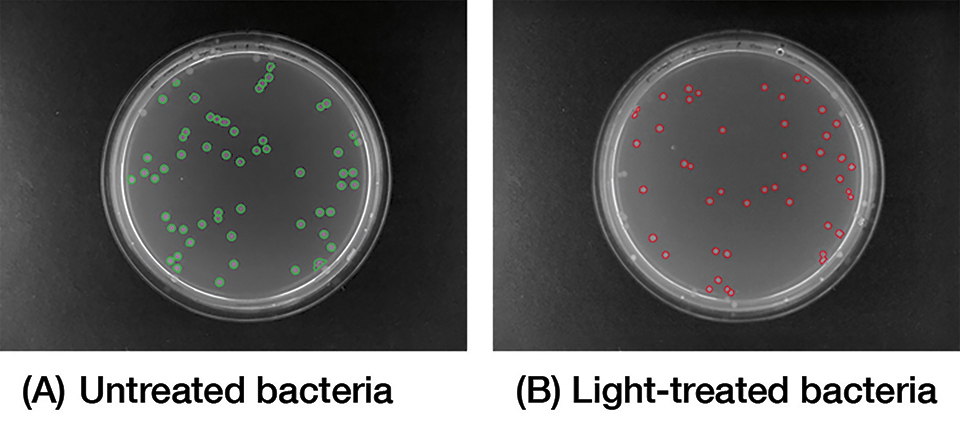
Figure 13: Image analysis of plated bacteria, either (A) untreated or (B) light-treated following blue light treatment
Thus, with blue light both issues were solved: On the one hand, bacterial growth and an accelerated epidermization can be blocked with higher doses of blue light, whereas on the other hand, low doses give rise to cell activation and stimulation of proliferation. Both findings help to improve wound management of chronic lesions, which was started to be verified in vivo showing first promising results confirming the outcomes found in vitro.
Outlook
The developed prototype is an ideal solution for a smart, wearable system for blue-light treatment of chronic wounds, such as diabetic ulcers. It further paves the way for the potential future commercialization of devices based on light therapy for monitoring wound healing. The application here is just one example of the many other imaginable applications enabled by the project, as the approach could also target other medical issues in the future.
The discovery and demonstration of the efficacy of blue light in both antibacterial functions and the activation of key cutaneous cells allowed to file two fundamental patents. The results also open the way for other important application opportunities that will address unmet needs, such as the disinfection of medical instruments and operating environments. It is important to note that MEDILIGHT was initially a human adventure. Several teams, several countries, and several skill-sets have joined forces to achieve a single goal: to create innovative healthcare solutions for tomorrow.
Acknowledgements:
MEDILIGHT is a European consortium funded within the H2020 Framework Program with an EU contribution of EUR 3.2 million started in February 2015, the project will come to an end in July 2018. The consortium builds on the competencies of the following seven partners: Technische Universität Berlin (TUB), Germany; URGO RID, France; Ruprecht-Karls-Universität Heidelberg, Germany; Centre Suisse d'Electronique et de Microtechnique SA (CSEM), Switzerland; SignalGeneriX Ltd, Cyprus; Microsemi Semiconductor Limited, United Kingdom; and AMIRES s.r.o., Czech Republic.
References:
[1] Frykberg RG, Banks J: Challenges in the Treatment of Chronic Wounds.
Advances in wound care, 4: 560-582, 2015
[2] Mamalis A, Garcha M, Jagdeo J: Light emitting diode-generated
blue light modulates fibrosis characteristics: fibroblast proliferation,
migration speed, and reactive oxygen species generation. Lasers in
surgery and medicine, 47: 210-215, 2015
[3] Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP, Hamblin MR:
Blue light for infectious diseases: Propionibacterium acnes,
Helicobacter pylori, and beyond? Drug resistance updates : reviews and
commentaries in antimicrobial and anticancer chemotherapy, 15:
223-236, 2012
[4] Moore P, Ridgway TD, Higbee RG, Howard EW, Lucroy MD: Effect of
wavelength on low-intensity laser irradiation-stimulated cell proliferation
in vitro. Lasers in surgery and medicine, 36: 8-12, 2005
[5] Gjodsbol, K, Christensen, JJ, Karlsmark, T, Jorgensen, B, Klein, BM,
Krogfelt, KA: Multiple bacterial species reside in chronic wounds:
a longitudinal study. International wound journal, 3: 225-231, 2006
[6] Becker A, Sticht C, Dweep H, van Abeelen FA, Gretz N, Oversluizen G:
Impact of blue LED irradiation on proliferation and gene expression of
cultured human keratinocytes. SPIE (Ed) SPIE BiOS San Francisco,
International Society for Optics and Photonics, 2015
[7] Becker A, Klapczynski A, Kuch N, Arpino F, Simon-Keller K,
De La Torre C, Sticht C, van Abeelen FA, Oversluizen G, Gretz N:
Gene expression profiling reveals aryl hydrocarbon receptor as a
possible target for photobiomodulation when using blue light.
Scientific reports, 6: 33847, 2016
[8] Khan AUM, Torelli A, Wolf I, Gretz N: AutoCellSeg: robust automatic
colony forming unit (CFU)/cell analysis using adaptive image
segmentation and easy-to-use post-editing techniques.
Scientific reports, 8: 7302