Technologies for Engineering an LED Light Closest to Sunlight
TRI-R is a next-generation LED light source made possible by Toshiba Materials technology and commercialization together with TOL STUDIO. Using natural light that recreates the solar spectrum, TRI-R suppresses glare, prevents the loss of object color, and gives fresher, clearer expression to textures and details. Based on professional research examining the relationship light has with humans and the environment, this is illumination technology that provides the world surrounding us with the true and real value of light. Masahiko Yamakawa, Senior Specialist of Toshiba Materials Co.LTD., presents how this technology was developed and applied in some projects.
White LED lighting sources were introduced into our social life over ten years ago and have become essential light devices. In comparison to classical lighting devices such as incandescent bulbs and fluorescent lamps, white LED lighting sources have all-round appealing powers; namely they have high energy efficiency, excellent life characteristic and low environmental burden to reduce running costs. And what is more, they can have unrestricted sizes, shapes and output powers to increase the freedom in design of the lighting devices. This is the reason why white LED lighting sources became popular in both facility and home lighting systems.
At present, in commercially available white LED lighting devices, blue lights emitted from LED chips are used as exciting light sources to make white light. In concrete terms, a part of blue light from an LED chip is used to be converted into yellow and red lights via appropriate phosphors. Then yellow and red lights are mixed up together with the remaining blue light to turn the mixture into a white light. On this occasion, because the blue light from an LED chip is higher in intensity than the yellow or red lights converted by phosphors, the white light produced by this method, in principle, tends to have a prominent blue region in the spectrum. Accordingly, some of those who have been long accustomed to sunlight, thermal radiation lights from incandescent bulbs make comments that they have little appreciation for such white LED light sources. These comments were the initial reason for developing and commercializing a new type of white LED light source.
The Technical Challenges
Two technical challenges exacerbate the development of an LED light source that has a specific light spectrum similar to that of the sunlight, with “Duplicate natural light out of artificial light sources!” as a theme.
The two major challenges are:
- Designing the ideal emission spectrum
- Holding down the degradation of energy efficiency
The first point aims to make an alternative system that does not employ blue light from an LED chip to make yellow or red light elements and to make a new phosphor design that renders broad visible light region in spectrum. The second point aims to develop new phosphor materials that have better quantum efficiency and better excitation light absorption rate and also to support process development in LED chips. The second point aims to develop new phosphor materials that have better quantum efficiency and better excitation light absorption rate and also to support process development in LED chips.
Commercialize a White LED Light Source that Duplicates Sunlight
Spectrum shows distribution of intensity of various lights alongside their wave length emitted from a certain light source. Figure 1 shows the light emission spectra of various lighting sources. Spectrum distributions of fluorescent lamps and white LED light bulbs show insufficient light intensity in some color regions. Especially, the white LED light bulbs have a distinctive feature that they have a precipitous intensity peak in the blue light region on account of the fact that they employ blue light as the exciting light source and what is more a transmitted part of that visible blue light is used as one of the mixing light elements of the white light. On the other hand, emission spectra of sunlight or an incandescent light bulb from thermal radiation are generally continuous.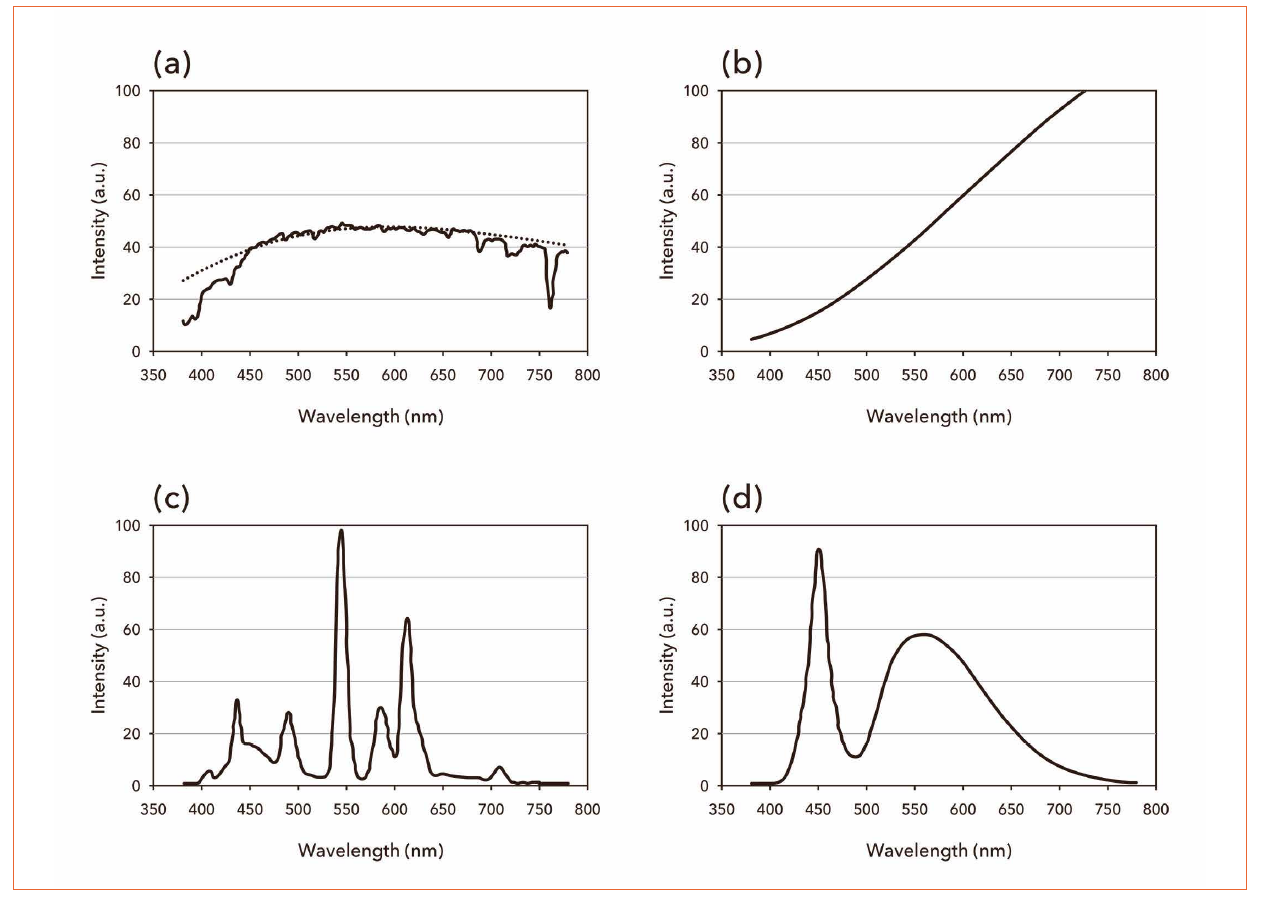 Figure 1: Comparison of the emission spectra of various lighting sources - (a) sunlight (dashed line: black body radiation), (b) incandescent light bulb, (c) fluorescent lamp, (d) commercial white LED
Figure 1: Comparison of the emission spectra of various lighting sources - (a) sunlight (dashed line: black body radiation), (b) incandescent light bulb, (c) fluorescent lamp, (d) commercial white LED
In a social life, we seldom have the opportunity to see directly illuminated light sources excepting display devices. We perceive an object, its colors, its brightness or its textures by receiving its reflected lights. This means that in case a light source had a deficient color region in the spectrum distribution, somewhat different senses will be created in visual recognition, which will not come out under usual light source with continuous spectrum distribution. In addition, a phenomenon that ample amount of light component in shorter wave length region causes trouble in visibility came to be a subject of study, standing on a fact that shorter the wave length, the light will be more easily scattered.
By the nature of light, spectrum pattern may vary unboundedly to make a similar color hue. Toshiba Materials Co. Ltd. has promoted the engineering development and commercialization of new type of white LED light sources that duplicate sunlight having the continuous spectrum distribution. To obtain a white LED light source with continuous spectrum distribution, there are two technical issues.
The exciting light should not be made one of the mixing light elements of the white light.
And it is necessary to develop a new type of phosphor that has an appropriate emission peak wavelength and emission half width in order not to make a deficient color region in the visible light region in the spectrum distribution.
The first issue was solved by employing a purple light emitting LED chip instead of blue light emitting ones. Figure 2 shows the schematic explanation of white light production principle and emission spectra. Moreover, a mechanism was developed to prevent the exciting light from transmitting by converting it into visible light by phosphor.
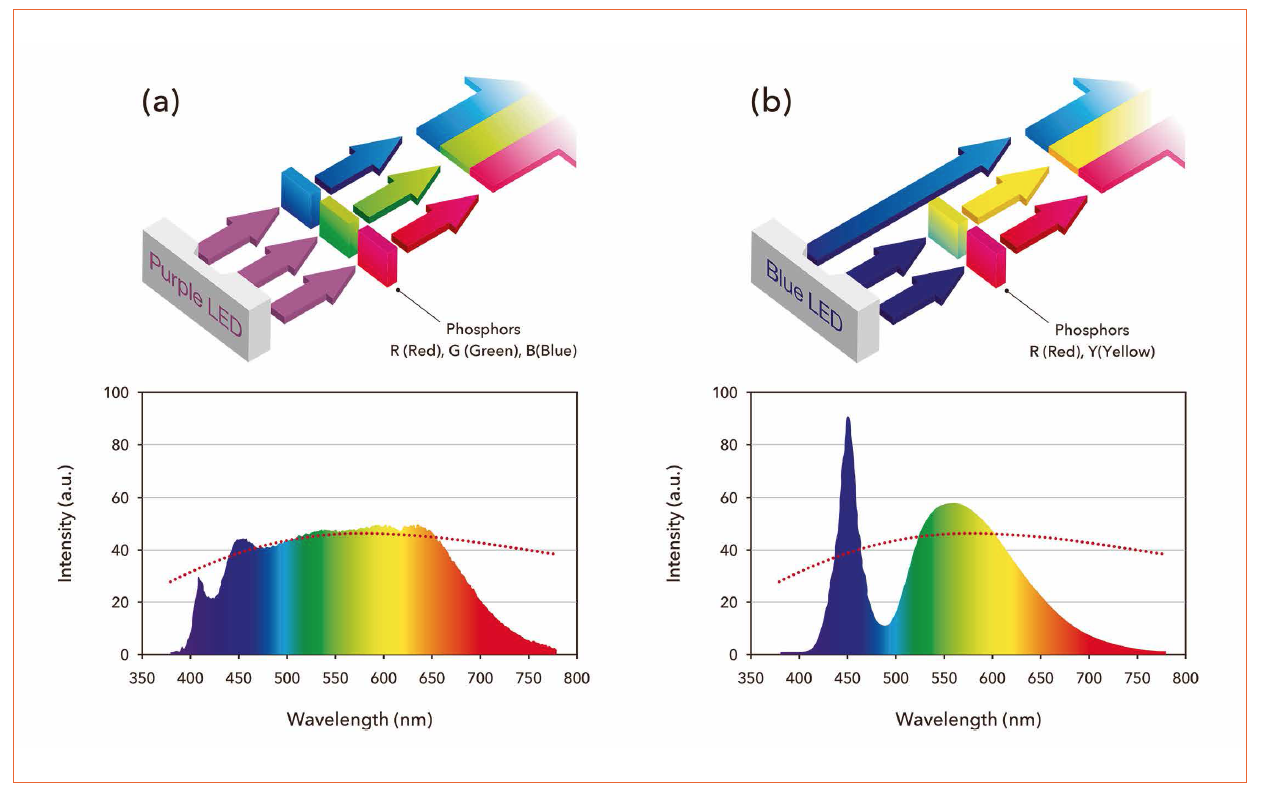 Figure 2: Comparison of the TRI-R approach with a commercial blue light pumped phosphor converted white LED - (a) TRI-R, (b) commercial white LED (Top: Principle of the production of white emission; Bottom: Emission spectra; dashed line: black body radiation)
Figure 2: Comparison of the TRI-R approach with a commercial blue light pumped phosphor converted white LED - (a) TRI-R, (b) commercial white LED (Top: Principle of the production of white emission; Bottom: Emission spectra; dashed line: black body radiation)
The second issue was solved by obtaining a new phosphor, on the basis material technology and optical simulation technology, by blending several kinds of phosphors so that the new one has an appropriate emission peak wavelength and emission half width. Obtaining a continuous spectrum distribution requires a close deployment of emission peaks along the wave length coordinate. A close deployment of emission peaks means the difference in energy levels of absorption and emission is small, suggesting causing loss in energy efficiency by mutual interference of adjacent phosphors. To cope with these difficulties, phosphor synthesis technology and phosphor layer structural design technology were applied.
Engineering Development of a White LED Light Source Excited by Purple Light
A purple light LED chip is used to make white light that duplicates the sunlight spectrum. The white LED light excited by a purple LED chip has a different emitting principle than that of those excited by blue LED chips.
New element technologies were required. Therefore, new phosphor material technology, phosphor layer formation technology, LED chip device technology and process technologies were developed. To produce the broad white spectrum, the design of the emission spectrum shape of each phosphor, the structure of the phosphor layer so as not to transmit the violet light, and the design to minimize the energy loss in the fluorescent layer are needed. Therefore, new phosphor material technology, phosphor layer formation technology, LED chip device technology and process technologies were developed.
Phosphor material technology
Phosphors are the ceramics-like powdery inorganic compounds with particle sizes varying from 10 to 30 μm. The phosphor materials are diluted doped rare earth elements. Phosphor materials include phosphate, silicate and oxonitrido-aluminosilicate, and as doping materials europium and cerium are often employed. When excited by some external stimulus or the purple light in this case, phosphors will emit a variety of colored lights from blue to red corresponding to the phosphor material and the doping material employed. Figure 3 shows the emission spectrum of various kinds of phosphors. A selection from these phosphors was blended to create the new phosphor mixture.
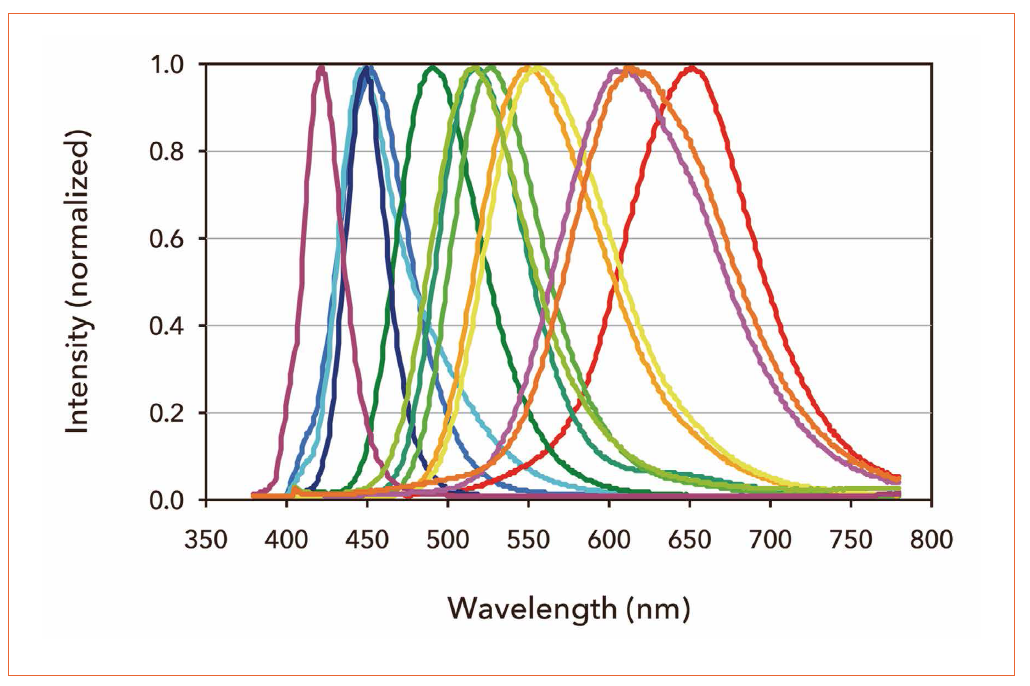 Figure 3: Emission spectra of phosphors for white LED excited by 405nm
Figure 3: Emission spectra of phosphors for white LED excited by 405nm
Phosphor materials are expected to have excellent optical properties, chemical properties and powder properties. The optical properties affect optical performances and energy efficiency. The chemical properties and powder properties affect energy efficiency and formation of production processes. Properties and the typically influenced performances are summed up in the table below.
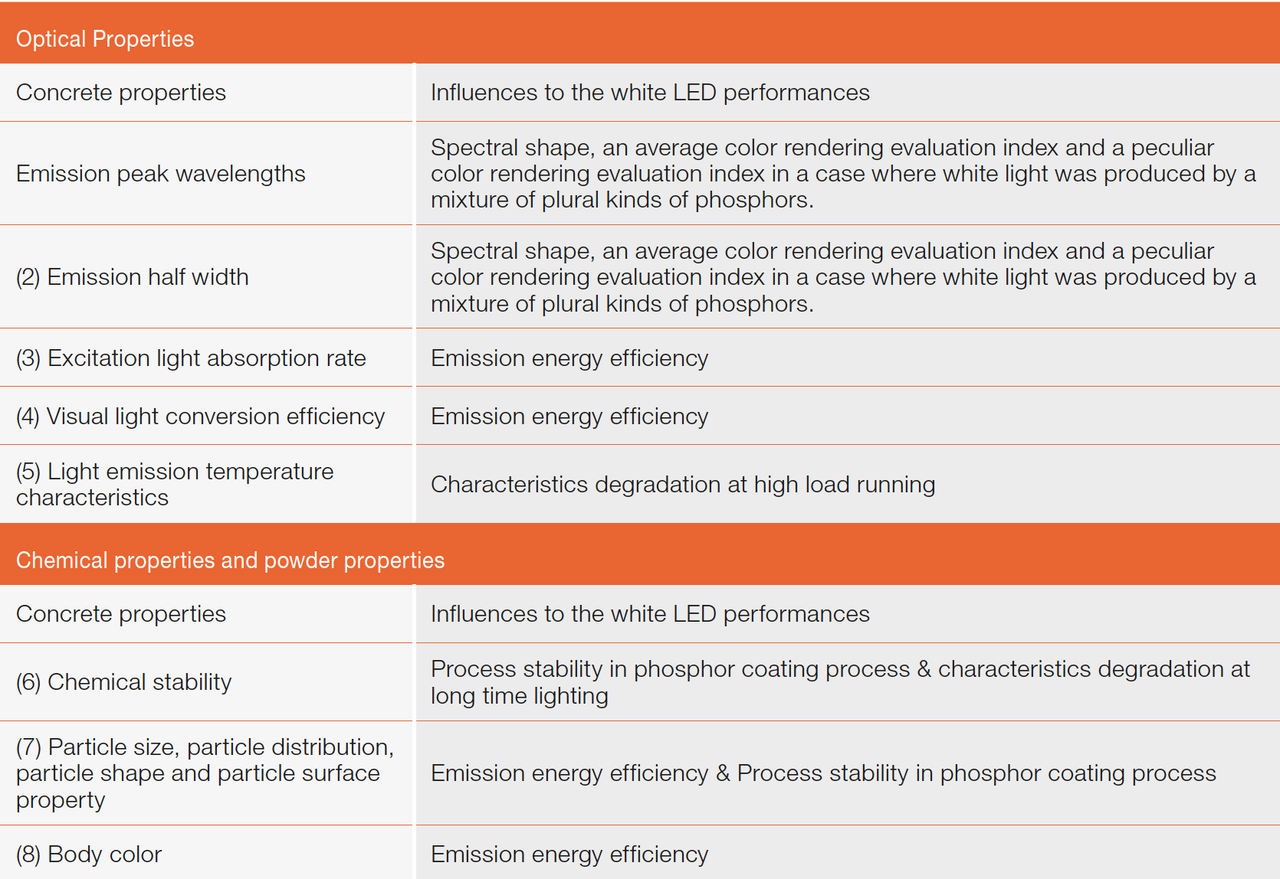 Table 1: Optical properties, chemical properties and powder properties and the typically influenced performances
Table 1: Optical properties, chemical properties and powder properties and the typically influenced performances
However, there are not a few antonymic relations in these properties. For example, excitation light absorption rate is generally inconsistent with visual light conversion efficiency. Moreover, although higher concentration of doping materials, which absorb exciting light, will be agreeable to improve absorption rate, color conversion rate will be degraded on account of the mutual interference between phosphor and doping materials. Further engineering development of phosphor synthesis was made to overcome this technical issue. The working items are listed in table 2.
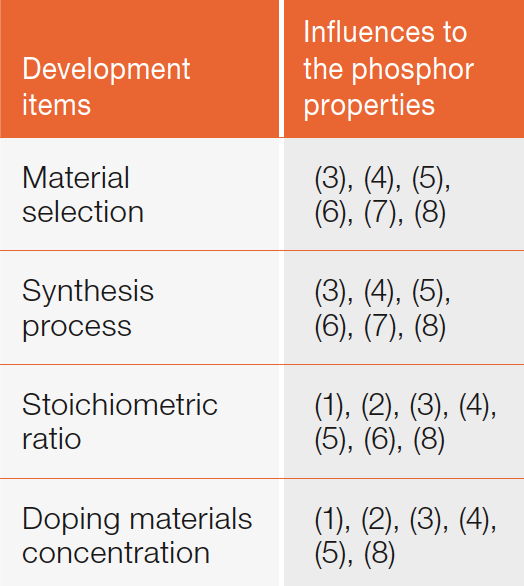 Table 2: Major development items of phosphor synthesis technology. The numbers indicate the concrete properties from Table 1 that are affected
Table 2: Major development items of phosphor synthesis technology. The numbers indicate the concrete properties from Table 1 that are affected
Let us pick up a blue phosphor as an example. Blue phosphor is represented by chemical formula M10(PO4)6Cl2, where M denotes alkaline earth metal and Eu doping element europium. This phosphor emits blue light effectively. This phosphor is made by burning the mixture of metallic oxides, metal carboxylate and phosphate metallic salts, which are the raw materials. In the burning process, target phosphor material will be produced by chemical reactions among molten raw materials, where the reactions will take place one after another in an orderly manner according to each one’s melting point and along with the temperature elevation. Control of chemical reactions among materials is indispensable to produce chemically stable crystals. To establish optimal synthesis process for producing phosphors, chemical reaction processes were investigated using both physical and chemical advanced analysis methods, namely X-ray diffraction analysis, elemental analysis and so on. Conditions for synthesis process were determined after investigation results were obtained.
Thus we have attained the optimal solution for the white LED lighting device after engineering development of every phosphor that makes up the device.
Phosphor layer formation technology
To install phosphor on the white LED, the LED is coated with the mixture of more than one phosphor mixed and dispersed in organic resin. Technical issues of this process are how to attain homogeneity of white light emission and how to prevent degradation of energy efficiency associated by mutual interference, which may be caused by the mixing of different kinds of phosphors. To attain homogeneity of white light emission, a lot of engineering effort was necessary to finally select the most appropriate transparent resin for the phosphor. Degradation of energy efficiency associated by a mutual interference is peculiar to the white LED that is coated with more than one phosphor. It was already explained above that phosphors, externally stimulated by an exciting light, attain broader emission at half width. The exciting light also has a certain wavelength range. Concretely speaking, a red phosphor will emit red light when excited by purple, blue or green exciting light. In the early stages of white LED light source development, a blue LED and a yellow phosphor were combined to produce a white light where the yellow phosphor was excited by blue light from the blue LED. On the other hand, in case of a high color rendering LED, where a blue LED and green and red phosphors were combined, the red phosphor, stimulated by exciting lights from a blue LED and a green phosphor, emits red light. In this instance, as the phosphor layers are made in a cascade structure, a mutual interference between phosphors is inevitable and results in energy loss. The purple light excited white LED light source, where every visible light is emitted from phosphors deployed in a cascade structure, also has the same issue with energy loss. To cope with this issue, a new phosphor paste coating process was developed, using a mixture of organic resin and phosphors to minimize the energy loss.
LED chip device and process technology
The emission wavelength of the light from a LED chip affects the energy efficiency. We have established the range of emission wavelength as 400 to 410 nm after studying the most appropriate range of emission wavelength of the light from the LED chip for our newly developed phosphors. Table 3 shows the results of the intense engineering developments.
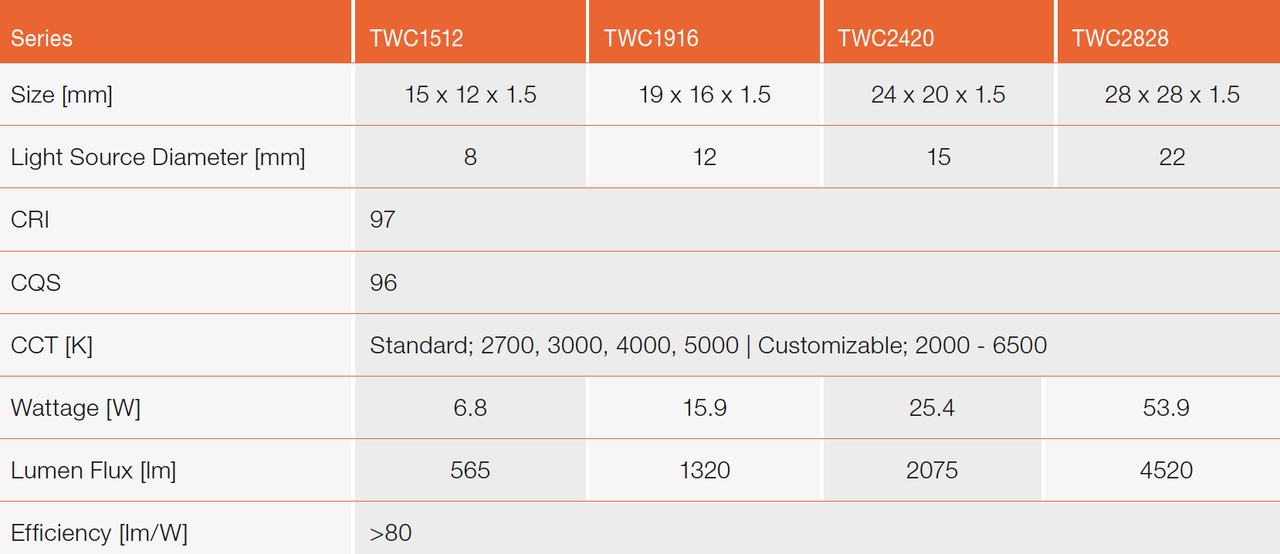 Table 3: Characteristics of TRI-R white LED
Table 3: Characteristics of TRI-R white LED
Applications to the Lighting Devices
White LEDs that use the new technology cover a wide range of color temperature varying from 2000K, or the color of a candle light, to 6500K, or the color of sunlight in a clear sky. And, what is more, they can duplicate the sunlight or natural light spectrum all over the said range of color temperature. Making use of these advantages, these white LEDs are employed as the alternatives to the incandescent light bulbs, as shadowless lamps or as lighting devices in display rooms.
These LEDs were employed in several new and innovative luminaires and projects, like the Lucellino LED of the Ingo Maurer Company, one of the German manufacturers of designed lighting devices. They have duplicated the features of incandescent light bulbs, namely the light spectrum, form and change of light colors when dimmed. In the lighting devices to illuminate the five central pieces of “Symbolism, Art in Europe from the Belle Époque to the Great War” held at the Palazzo Reale, Milan from 3rd February to 5th June 2016, they accurately reproduced the color, texture and 3D appearance of the pictures (Figure 4).
 Figure 4: Comparison of the difference in a painting is seen - (a) TRI-R, (b) commercial white LED (“Symbolism.
Figure 4: Comparison of the difference in a painting is seen - (a) TRI-R, (b) commercial white LED (“Symbolism.
Art in Europe from the Belle Époque to the Great War”, Palazzo Reale, Milan from 3rd February to 5th June 2016, La Giovinezza (Youth) by Giorgio Kienerk, 1902, Pavia, Musei Civici)
The technology is also applied in the Pinacoteca Ambrosiana, Milan. The museum is famous for housing a collection of pictures typified by “Codice Atlantico” of Leonardo da Vinci, “Madonna del Padiglione” of Botticelli and “Canestra di frutta” of Caravaggio. Finally, Shadowless lamp in Japan appreciated the characteristics for the hue and reproducibility at a higher color temperature, excellent visibility and the ability to reduce effects of visual burden of the operator, comparable to that of conventionally used halogen lamps.
Conclusion
In recent years, relations between light and health are being discussed. Human beings have utilized lights as one of the means of living throughout their history. Light sources, in the early days were, were limited to natural light such as sunlight and fire flames. Accordingly, our bodily functions were adapted to those natural lights. Blue lights are absorbed in the retina and its signal is transmitted to the biological central clock (suprachiasmatic nucleus) to control biological clocks. In the morning, exposure to sunlight resets the biological rhythm. On the other hand, if exposed to light at night, the biological clock may go off kilter and sleep will be negatively affected. Because of this it is preferable to choose human friendly light, or it is important to avoid bluish lights at night. Research on the impact of lights on the human body are advancing throughout the world; it will be clarified and include sufficient medical verification.
Illuminations shine lights on various objects. Man recognizes objects by learning their features through sight which is created by the intensities or wavelengths of reflected lights. After that, man can respond in identifications or in emotions. The faculty of seeing something includes seeing beautifully and correctly. To enhance this faculty it is necessary to design and offer adequate illumination lights with low impact to visual organs and the nerve system of the human body.

