The Perspective of Bio-Hybrid White Light-Emitting Diodes Based on Rubber-Like Down- Converting Coatings
At first, white LED technology seems to be mature. But in truth, the technology is still far from being perfect and research on all LED materials and components is ongoing. At the LpS 2016 the research of Rubén D. Costa, Lukas Niklaus, Katharina Weber and Prof. Uwe Sonnewald from the University of Erlangen-Nuremberg (FAU) was honored with the LED professional Scientific Award. These authors investigated the use of organic light conversion materials for white LEDs. Their research shows that these bio-hybrid white LEDs could lower costs and improve light quality. The article describes the current status of the research and future perspectives.
White hybrid light-emitting diodes (WHLEDs) consist of an inorganic blue-emitting LED that is coated with organic down-converting layers, which are easy-to-prepare and eco-friendly. The current challenge of WHLEDs is to find a universal packing matrix and stable organic down-converting compounds to maximize the device performance. In this contribution, we show the straightforward fabrication of WHLEDs by designing a novel sealing-free gel that transforms into a rubber-like material under moderate vacuum conditions without using any cross-linking and UV- or thermal-curing methods. This approach allows to compare different down-converting organic materials in the same environment - i.e., the same packing matrix, blue-emitting LED source, and environmental conditions. In detail, laser dyes, carbon nanodots, luminescent polymers, coordination complexes, and fluorescent proteins were directly compared showing the prospect of WHLEDs with rubber-based coatings.
Properties of the current WHLED generation:
- Bio-WHLEDs, featuring 50 lm/W with a loss of less than 10% during 100 h under operation conditions and
- WHLEDs based on coordination complexes, showing stabilities of more than 1000 h - extrapolated 4000 h - with luminous efficiencies of 100 lm/W and no color degradation. As such, our work provides a clear prospect of this emerging technology
Introduction
White solid-state lighting sources for outdoor and indoor applications are currently in the focus of both academic and industrial research. On one hand, all-inorganic white light-emitting diodes (WLEDs) are meant to imminently replace inefficient and environmentally harmful incandescent and fluorescent lamps [1-4]. On the other hand, WLEDs exhibit a more complex architecture than the monochromatic LEDs due to the lack of intrinsic white light-emitting semiconductors. Today the most mature approach is to coat the chip of a blue-emitting LED with down-converting materials such as yellow-emitting inorganic phosphors - e.g., YAG:Ce3+ derivatives - to convert the LED emission into white light [5,6].
The main bottlenecks:
- High cost concerning production and materials that are based on rare-earth components
- Lack of recycling procedures
- Poor color quality of the lighting devices, since stable and efficient inorganic phosphors that emit in the deep-red region are still missing [3,4,7-11]
In this regard, both the photonics EU commission and the US Department of Energy are encouraging the development of novel non-rare earth metal and non-toxic down-converters, targeting four major aspects.
The four major aspects:
- Photoluminescence quantum yields higher than 95% (green and red regions)
- Thermal stability up to 150 °C
- Narrow emission bands with full widths at half maximum (FWHM) of 30-70 nm
- Color shift over time of Δu’v’ < 0.002 over lifetime
- Flux density saturation of at least 95% [2,4]
Therefore, the scientific community has seriously started to explore several approaches to implement environmentally friendly down-converting materials into white hybrid LEDs (WHLEDs), which are heralded as the next generation of low- and mid-power WLEDs if the device performance equals the current status (Figure 1 - [12-39]). In this new field, one of the main challenges is the packing system. For instance, the first WHLEDs were prepared with UV- and thermal-curing polymers like silicones that promote the degradation of the down-converting compounds upon coating fabrication, and typically compromise the spatial color distribution due to phase separation issues between the matrix and the down-converting compounds [12,14,15,35-39].
Recent matrices are based on:
- Metal-organic frameworks (MOFs), in which down-converting laser dyes can be adsorbed [24-26]
- Cellulose, in which inorganic and graphitic quantum dots are embedded, have been demonstrated (Figure 1) [27,29]
They all have in common, that the used organic down-converters show a general good thermal- and photo-stability along with high photoluminescence quantum yields, an ease of color tunability covering the whole visible and NIR spectra, and well-known recycling protocols. Therefore, these WHLEDs feature an excellent color quality, but still with a low stability ranging from a few minutes to several hundreds of hours under continuous working conditions [12-39]. In addition, it is difficult to compare these matrix approaches due to incompatibility issues with the down-converting compounds. In this contribution, we present our novel approach based on the design of a rubber-like coating in which - for the first time - a wide variety of well-known environmentally friendly commercially available down-converting materials like fluorescent proteins, laser dyes, carbon quantum dots, polymers, and coordination complexes (Figure 2) are implemented and directly compared in WHLEDs, as explained in the following sections [30,31,39].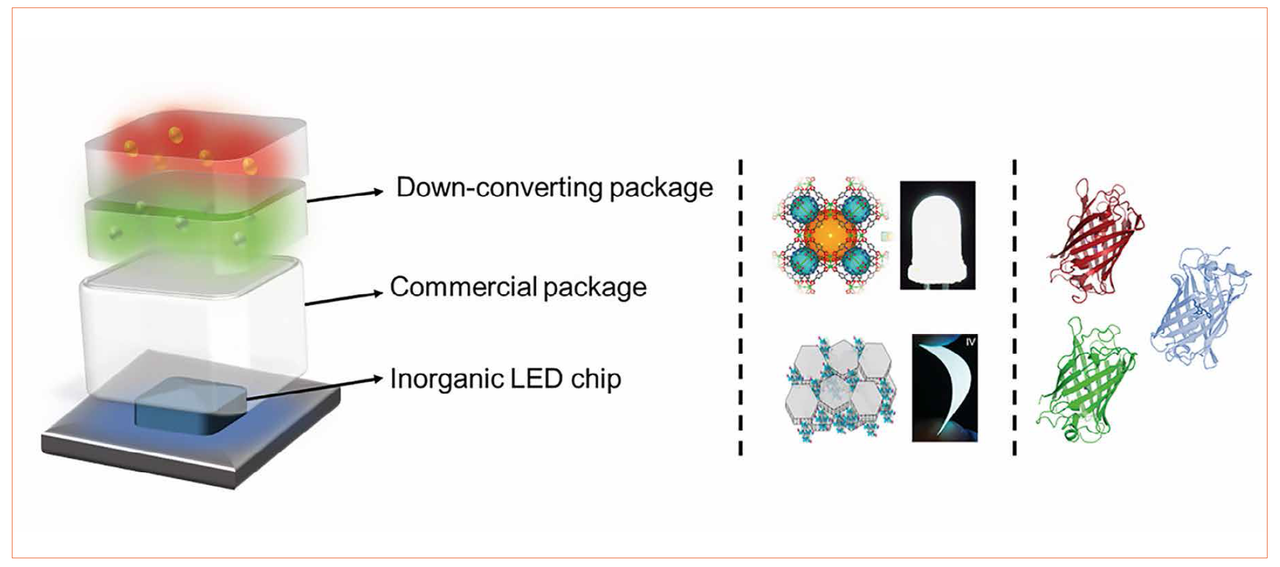
Figure 1: Left - Representation of an archetypal WHLED. Center - Recently reported WHLEDs based on MOFs- and cellulose-based approaches (top and bottom, respectively). Right - schematic representation of the green, red, and blue emitting proteins
General Information about Rubber-Like Down- Converting Coatings
In stark contrast to the state-of-the-art packing fabrication, our rubber-like coatings are not based on UV- or thermal-curable cross linking approaches or adsorption procedures into the matrix. Here, both gels and rubbers are prepared by simply mixing of branched and linear polyethylene oxides. The viscous properties of the formed gels allow an excellent handling for coating purposes. As an example, Figure 2 shows different 3D forms like a spoon, knife, and fork coated with the gel-like material. Subsequent treatment of the soft doctor-bladed films under vacuum conditions for less than 1 h at room temperature leads to the final rubber-like material exhibiting a transmittance and refractive index superior to 95 % and 1.8, respectively. But there are some more important aspects.
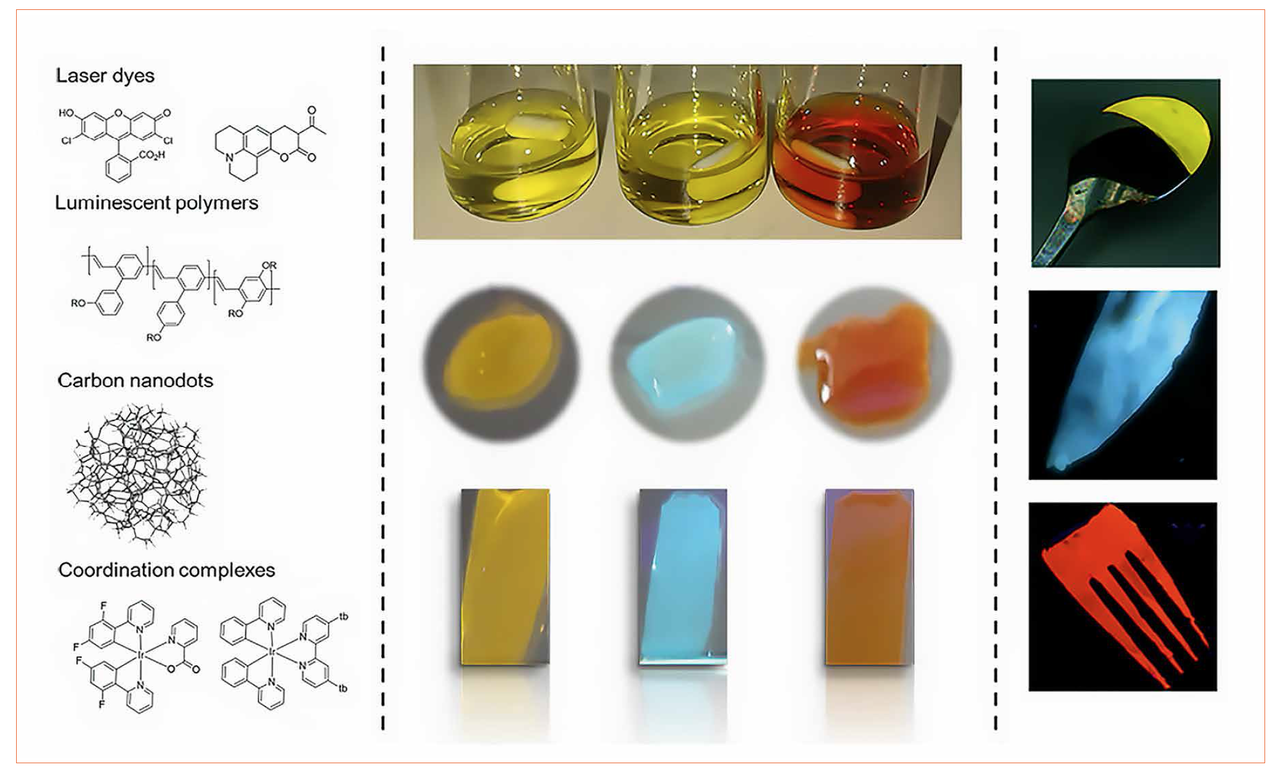 Figures 2: Left - chemical structures of the luminescent compounds. Center - pictures of the coordination complex-based gels (room light, with a magnetic stirrer, top), the corresponding gel drops (middle), and the rubber-like materials (bottom) under UV excitation (310 nm, 8 W). Right - pictures of exemplary luminescent rubber-like materials deposited onto irregular 3D surfaces (λexc = 310 nm), such as spoon, knife, and fork
Figures 2: Left - chemical structures of the luminescent compounds. Center - pictures of the coordination complex-based gels (room light, with a magnetic stirrer, top), the corresponding gel drops (middle), and the rubber-like materials (bottom) under UV excitation (310 nm, 8 W). Right - pictures of exemplary luminescent rubber-like materials deposited onto irregular 3D surfaces (λexc = 310 nm), such as spoon, knife, and fork
Three more important aspects:
- Luminescent features of the down-converting compounds are preserved in both gel and rubbers compared to those in solution
- Rheological features can be easily controlled by the matrix components allowing any coating technique like R2R, 3D printing, etc.
- All luminescent compounds embedded in the rubbers show excellent storage stabilities over months and thermal stabilities up to 100 °C [30,31,39]
Comparing White Hybrid Light-Emitting Diodes Based on Rubber-Like Down-Converting Coatings
As above-explained, a commercial blue-emitting LED is coated with a multi-layered down-converting coating that features a bottom-up energy transfer process. In particular, the inorganic LED excites the bottom down-converting layer, which then emits light being partially absorbed from the next layer that further emits light (Figure 1). The combination of the emission from the LED and the several layers leads to a white emitting LED. By a simple change of the layer thickness, the emission spectrum can be adjusted to cover the whole visible spectrum. As starting conditions, the performance of the WHLEDs was measured under dried N2 atmosphere at different applied currents. Afterwards, the devices were driven at the optimum performance concerning luminous efficiency and white color quality under inert conditions. Finally, the most stable devices were subsequently measured under ambient conditions (Figure 3). This allows us to provide a direct comparison between the different down-converting compounds. In detail, WHLEDs with different laser dyes feature and initial excellent color rendering index > 95 but a low color stability of a few hours even under inert atmosphere (Figure 3). Therefore, no further measurements were carried out with these class of compounds. The WHLEDs based on carbon nanodot coatings exhibit an initial low luminous efficiency of around 2 lm/W (Figure 3). The color quality changes after around 20 h due to the evolution of a new red-emitting component. During the subsequent 30 h under ambient conditions, the electroluminescence spectra quickly evolved until a more balanced contribution in the yellow and red parts is present, but with a more prominent blue component, while the luminous efficiency further reduces up to values of around 1 lm/W (Figure 3). After this point, the electroluminescence spectrum is constant, but the device features a low luminous efficiency. It is important to note that the changes in the electroluminescence spectrum are related to changes in the molecular structure, which is still under hot debate.
The most efficient WHLEDs were based on a thin luminescent polymer-based down-converting coating, showing white light that is stable over about 200 h under inert atmosphere with a luminous efficiency of around 200 lm/W (Figure 3). A subsequent measurement of the same polymer-based WHLED under ambient conditions showed that, unfortunately, the emission of the polymer is immediately damaged by the well-known photo-assisted oxidation process, as both the color quality and the luminous efficiency declined, which is already reported in the literature [15,40-42].
Next, the protein-based rubber-like materials were applied as down-converting coatings in order to present the first Bio-WHLEDs (Figure 3). The latter shows a perfect coverage of the whole visible spectrum with a luminous efficiency of 50 lm/W. Moreover, the loss of efficiency under working conditions during 100 h is less than 10% independently of the ambient conditions, highlighting the prospect of this type of materials (Figure 3).
Finally, we fabricated WHLEDs with thin coordination complex based coatings (Figure 3). Similar to the Bio-WHLEDs, these devices show an excellent stability in terms of both color quality and luminous efficiency (100 lm/W under N₂ atmosphere and 70 lm/W under ambient conditions) for several hundreds of hours. In addition, these WHLEDs show a remarkable stability in terms of color and efficiency over more than 1000 hours (extrapolated to 4000 h) even under ambient operation conditions.[31] Interestingly, the luminous efficiency is immediately reduced or increased when transferring the CC-WHLED from N₂ to ambient conditions and vice versa (Figure 3), which is related to the well-known phosphorescence quenching by oxygen.
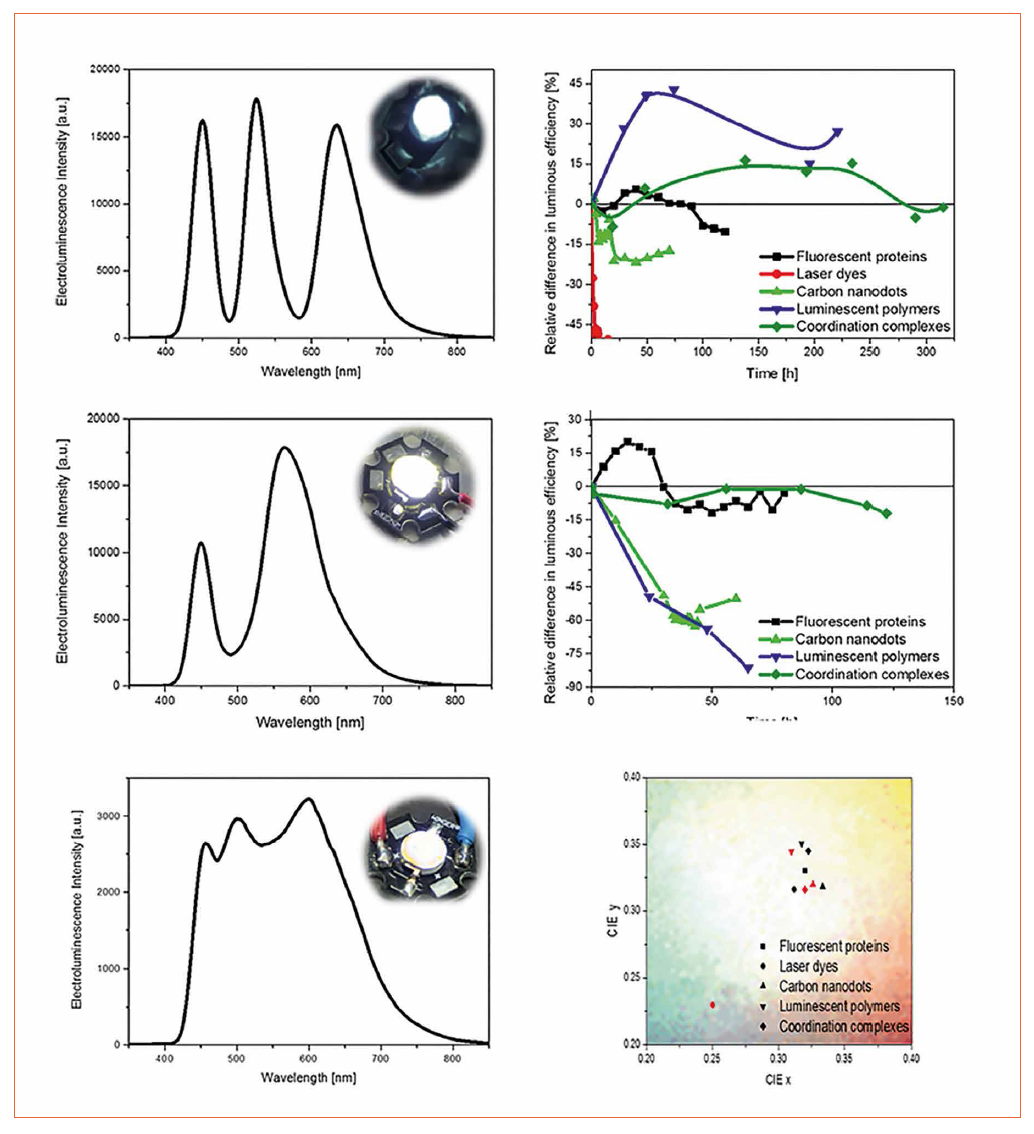 Figure 3: Left - Electro-luminescence spectra of the most promising WHLEDs - i.e., protein- (top), polymer- (center), and coordination complex-based WHLEDs (bottom). The insets show the pictures of the WHLEDs under operation conditions. Right - Change of the luminous efficiency over time for several WHLEDs under N2 conditions (top) and under O2 atmosphere (center). The CIE coordinates of the WHLEDs (bottom) at the beginning (black) and after 70% of the luminous efficiency is reached (red) are depicted
Figure 3: Left - Electro-luminescence spectra of the most promising WHLEDs - i.e., protein- (top), polymer- (center), and coordination complex-based WHLEDs (bottom). The insets show the pictures of the WHLEDs under operation conditions. Right - Change of the luminous efficiency over time for several WHLEDs under N2 conditions (top) and under O2 atmosphere (center). The CIE coordinates of the WHLEDs (bottom) at the beginning (black) and after 70% of the luminous efficiency is reached (red) are depicted
Conclusions and Outlook
Despite the fact that Bio-WHLEDs exhibit a great potential to overcome most of the above mentioned bottlenecks. However, Bio-WHLEDs have also still their limitations.
Limitations of Bio-WHLEDs:
- Denaturation of the proteins owing to oxidative stress, which leads to a loss of color quality
- High thickness in the millimeter regime due to the energy-transfer cascade architecture
- Low thermal stability of up to 100 °C of the matrix as well as the proteins
However, fluorescent proteins fulfilled all the targets highlighted by the US and EU departments in terms of high photoluminescence quantum yields, narrow emission, no spectra shift upon degradation, and high photon density saturation as recently demonstrated in protein-based lasers [2,3,43]. In addition, their production is cheap and can be realized around the whole world using well-known up-scaling protocols. Finally, they are non-toxic and do not produce any hazard residues. All these aspects clearly point out the potential of this material for future developments of Bio-WHLEDs.
This is even clearer when the device performances are compared. For instance, laser dyes lack stability concerning thermal and UV-treatment and should therefore be replaced by new designed compounds, while carbon nanodots exhibit an initial low luminous efficiency of around 2 lm/W that is related to the poor photoluminescence quantum yields in solid-state and the changes of the electroluminescence spectrum over long periods of time. It is important to note that a proper design of carbon nanodots with, for example, organosilane outer substituents and/ or encapsulated carbon QDs might solve both problems as very promising results have been recently shown [44]. In the case of luminescent polymers, a further encapsulation system will be necessary for improving their lifespan under operating conditions with the shortcoming of a less user-friendly fabrication process. Even though coordination complex-based WHLEDs show no need for an extra encapsulation in terms of stability - i.e., when comparing the stability of our WHLEDs with the state-of-art stability that is around a few hundreds of hours - it is encouraged the design of efficient and low-cost coordination complexes, since the price of Iridium is currently too high for industrial purposes [45].
Finally, the milestone of WHLEDs is the development of down-converting encapsulation systems for high-powerful LED arrays, which hold high operation temperatures. Currently, we are working on different strategies to enhance the thermal- and photo-stability by a proper design of the fluorescent proteins along with modifications of the matrix components. Finding solutions for these roadblocks is crucial to bring the Bio-WHLED from the laboratory scale to industrial applications.
References:
[1] R. Haitz, J. Y. Tsao, phys. stat. sol. (a) 2011, 208, 17
[2] Federation of National Manufacturers Association for Luminaires and Electrotechnical Components for Luminaires in the European Union & European Lamp Companies Federation, The European Lighting Industry’s Considerations Regarding the need for an EU Green Paper On Solid State Lighting 2011
[3] U.S. Department of Energy, Manufacturing Roadmap Solid-State Lighting Research and Development 2014
[4] U.S. Department of Energy, Solid-State Lighting R&D Plan 2015
[5] S. Nakamura, T. Mukai, M. Senoh, S.-i. Nagahama, N. Iwasa, J. Appl. Phys. 1993, 74, 3911
[6] S. Nakamura, J. Crys. Growth 1999, 201, 290
[7] H. A. Hoppe, Angew. Chem. Int. Ed. 2009, 48, 3572
[8] M. Shang, C. Li, J. Lin, Chem. Soc. Rev. 2014, 43, 1372
[9] Y. Wang, G. Zhu, S. Xin, Q. Wang, Y. Li, Q. Wu, C. Wang, X. Wang, X. Ding, W. Geng, J. Rare Earth 2015, 33, 1
[10] P. F. Smet, A. B. Parmentier, D. Poelman, J. Electrochem. Soc. 2011, 158, R37
[11] V. K. Khanna, Fundamentals of solid-state lighting: LEDs, OLEDs, and their applications in illumination and displays, CRC Press, Boca Raton, FL 2014
[12] G. Heliotis, G. Itskos, R. Murray, M. D. Dawson, I. M. Watson, D. D. C. Bradley, Adv. Mater. 2006, 18, 334
[13] G. Heliotis, P. N. Stavrinou, D. D. C. Bradley, E. Gu, C. Griffin, C. W. Jeon, M. D. Dawson, Appl. Phys. Lett. 2005, 87, 103505
[14] E. Gu, H. X. Zhang, H. D. Sun, M. D. Dawson, A. R. Mackintosh, A. J. C. Kuehne, R. A. Pethrick, C. Belton, D. D. C. Bradley, Appl. Phys. Lett. 2007, 90, 31116
[15] I. O. Huyal, U. Koldemir, T. Ozel, H. V. Demir, D. Tuncel, J. Mater. Chem. 2008, 18, 3568
[16] O.-H. Kim, S.-W. Ha, J. I. Kim, J.-K. Lee, ACS Nano 2010, 4, 3397
[17] M. Stupca, O. M. Nayfeh, T. Hoang, M. H. Nayfeh, B. Alhreish, J. Boparai, A. AlDwayyan, M. AlSalhi, J. Appl. Phys. 2012, 112, 74313
[18] W.-S. Song, H. Yang, Chem. Mater. 2012, 24, 1961
[19] N. J. Findlay, J. Bruckbauer, A. R. Inigo, B. Breig, S. Arumugam, D. J. Wallis, R. W. Martin, P. J. Skabara, Adv. Mater. 2014, 26, 7290
[20] N. J. Findlay, C. Orofino-Peña, J. Bruckbauer, S. E. T. Elmasly, S. Arumugam, A. R. Inigo, A. L. Kanibolotsky, R. W. Martin, P. J. Skabara, J. Mater. Chem. C 2013, 1, 2249
[21] D. Di Martino, L. Beverina, M. Sassi, S. Brovelli, R. Tubino, F. Meinardi, Sci. Rep. 2014, 4, 4400
[22] J. Chen, W. Liu, L.-H. Mao, Y.-J. Yin, C.-F. Wang, S. Chen, J. Mater. Sci. 2014, 49, 7391
[23] C. Sun, Y. Zhang, K. Sun, C. Reckmeier, T. Zhang, X. Zhang, J. Zhao, C. Wu, W. W. Yu, A. L. Rogach, Nanoscale 2015, 7, 12045
[24] Q. Gong, Z. Hu, B. J. Deibert, T. J. Emge, S. J. Teat, D. Banerjee, B. Mussman, N. D. Rudd, J. Li, J. Am. Chem. Soc. 2014, 136, 16724
[25] Y. Lu, B. Yan, Chem. Comm. 2014, 50, 15443
[26] Y. Cui, T. Song, J. Yu, Y. Yang, Z. Wang, G. Qian, Adv. Funct. Mater. 2015, 25, 4796
[27] H. Tetsuka, A. Nagoya, R. Asahi, J. Mater. Chem. C 2015, 3, 3536
[28] H. Tetsuka, A. Nagoya, T. Fukusumi, T. Matsui, Adv. Mater. 2016, 28, 4756
[29] D. Zhou, H. Zou, M. Liu, K. Zhang, Y. Sheng, J. Cui, H. Zhang, B. Yang, ACS Appl. Mater. Interfaces 2015, 7, 15830
[30] M. D. Weber, L. Niklaus, M. Pröschel, P. B. Coto, U. Sonnewald, R. D. Costa, Adv. Mater. 2015, 27, 5493
[31] L. Niklaus, H. Dakhil, M. Kostrzewa, P. B. Coto, U. Sonnewald, A. Wierschem, R. D. Costa, Mater. Horiz. 2016 DOI: 10.1039/C6MH00038J
[32] P.-C. Shen, M.-S. Lin, C.-F. Lin, Sci. Rep. 2014, 4, 5307
[33] E.-P. Jang, W.-S. Song, K.-H. Lee, H. Yang, Nanotechnology 2013, 24, 45607
[34] C.-F. Lai, C.-J. Chang, C.-L. Hsieh, Y.-L. Chen, C.-S. Tuan, Opt. Lett. 2013, 38, 4082
[35] K. Chang, X. Men, H. Chen, Z. Liu, S. Yin, W. Qin, Z. Yuan, C. Wu, J. Mater. Chem. C 2015, 3, 7281
[36] F. Hide, P. Kozodoy, S. P. DenBaars, A. J. Heeger, Appl. Phys. Lett. 1997, 70, 2664
[37] C. Zhang, A. J. Heeger, J. Appl. Phys. 1998, 84, 1579
[38] M. T. Sajjad, P. P. Manousiadis, H. Chun, D. A. Vithanage, S. Rajbhandari, A. L. Kanibolotsky, G. Faulkner, D. O’Brien, P. J. Skabara, I. D. W. Samuel, G. A. Turnbull, ACS Photonics 2015, 2, 194
[39] M. D. Weber, M. Pröschel, P. B. Coto, U. Sonnewald, R. D. Costa, Rubber-like material for the immobilization of proteins and its use in lighting, diagnosis and biocatalysis. EP 15173026.4-1408
[40] G. Yu, Y. Cao, M. Andersson, J. Gao, A. J. Heeger, Adv. Mater. 1998, 10, 385
[41] J. Fang, P. Matyba, L. Edman, Adv. Funct. Mater. 2009, 19, 2671
[42] A. Asadpoordarvish, A. Sandström, L. Edman, Adv. Eng. Mater. 2016, 18, 105
[43] M. C. Gather, S. H. Yun, Nature communications 2014, 5, 5722
[44] H. Yoo, H. S. Jang, K. Lee, K. Woo, Nanoscale 2015, 7, 12860
[45] D. Volz, M. Wallesch, C. Fléchon, M. Danz, A. Verma, J. M. Navarro, D. M. Zink, S. Bräse, T. Baumann, Green Chem. 2015, 17, 1988

