MIT Team Coaxes Polymers To Line Up, Transforming Them Into Materials That Could Dissipate Heat
MIT team has found a way to transform the most widely used polymer, polyethylene, into a material that conducts heat just as well as most metals, yet remains an electrical insulator.
The new process causes the polymer to conduct heat very efficiently in just one direction, unlike metals, which conduct equally well in all directions. This may make the new material especially useful for applications where it is important to draw heat away from an object, such as a computer processor chip. The work is described in a paper published on March 7 in Nature Nanotechnology.
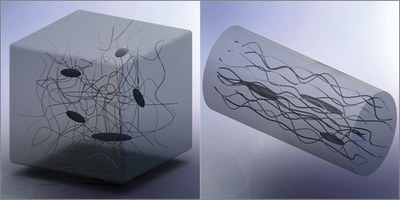
The key to the transformation was getting all the polymer molecules to line up the same way, rather than forming a chaotic tangled mass, as they normally do. The team did that by slowly drawing a polyethylene fiber out of a solution, using the finely controllable cantilever of an atomic force microscope, which they also used to measure the properties of the resulting fiber.
This fiber was about 300 times more thermally conductive than normal polyethylene along the direction of the individual fibers, says the team’s leader, Gang Chen, the Carl Richard Soderberg Professor of Power Engineering and director of MIT’s Pappalardo Micro and Nano Engineering Laboratories.
The high thermal conductivity could make such fibers useful for dissipating heat in many applications where metals are now used, such as solar hot water collectors, heat exchangers and electronics.
Chen explains that most attempts to create polymers with improved thermal conductivity have focused on adding in other materials, such as carbon nanotubes, but these have achieved only modest increases in conductivity because the interfaces between the two kinds of material tend to add thermal resistance. “The interfaces actually scatter heat, so you don’t get much improvement,” Chen says. But using this new method, the conductivity was enhanced so much that it was actually better than that of about half of all pure metals, including iron and platinum.
Producing the new fibers, in which the polymer molecules are all aligned instead of jumbled, required a two-stage process, explains graduate student Sheng Shen, the lead author of the paper. The polymer is initially heated and drawn out, then heated again to stretch it further. “Once it solidifies at room temperature, you can’t do any large deformation,” Shen says, “so we heat it up twice.”
Even greater gains are likely to be possible as the technique is improved, says Chen, noting that the results achieved so far already represent the highest thermal conductivity ever seen in any polymer material. Already, the degree of conductivity they produce, if such fibers could be made in quantity, could provide a cheaper alternative to metals used for heat transfer in many applications, especially ones where the directional characteristics would come in handy, such as heat-exchanger fins (like the coils on the back of a refrigerator or in an air conditioner), cell-phone casings or the plastic packaging for computer chips. Other applications might be devised that take advantage of the material’s unusual combination of thermal conductivity with light weight, chemical stability and electrical insulation.
So far, the team has just produced individual fibers in a laboratory setting, Chen says, but “we’re hoping that down the road, we can scale up to a macro scale,” producing whole sheets of material with the same properties.
Ravi Prasher, an engineer at Intel, says that “the quality of the work from Prof. Chen’s group has always been phenomenal,” and adds that “this is a very significant finding” that could have many applications in electronics. The remaining question, he says, is “how scalable is the manufacturing of these fibers? How easy is it to integrate these fibers in real-world applications?”
This work, which also included Chen’s former graduate students Asegun Henry and Jonathan Tong, was supported by the National Science Foundation and the Department of Energy’s Office of Basic Energy Sciences.