New Technique Makes LEDs Brighter, More Resilient
Researchers from North Carolina State University have developed a new processing technique that makes light emitting diodes (LEDs) brighter and more resilient by coating the semiconductor material gallium nitride (GaN) with a layer of phosphorus-derived acid.
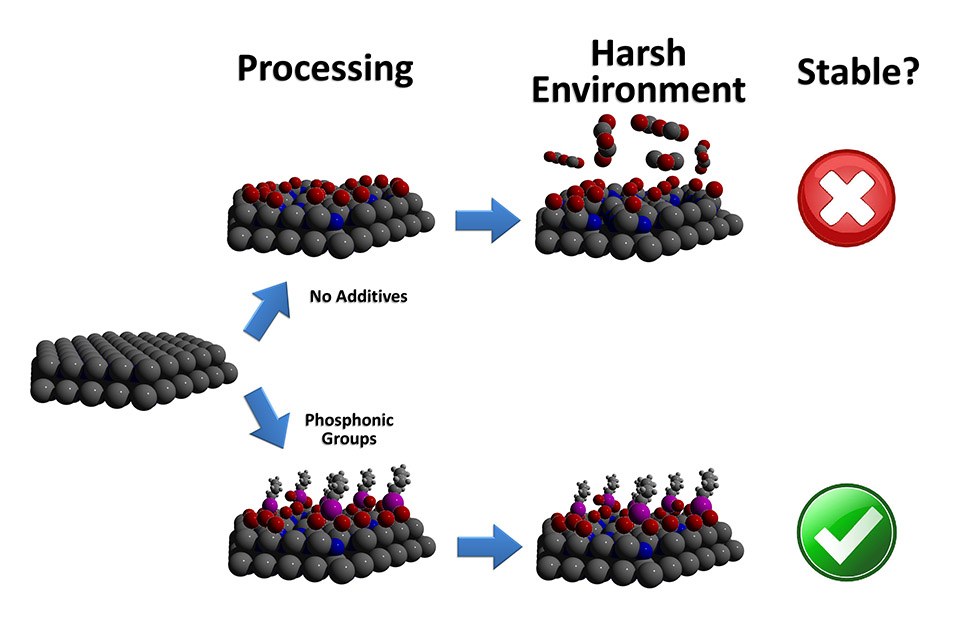
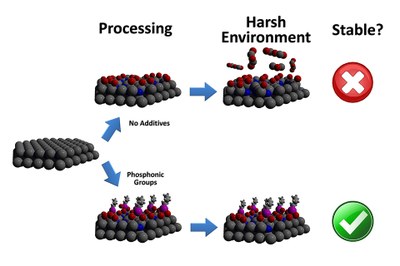
“By coating polar GaN with a self-assembling layer of phosphonic groups, we were able to increase luminescence without increasing energy input,” says Stewart Wilkins, a Ph.D. student at NC State and lead author of a paper describing the work. “The phosphonic groups also improve stability, making the GaN less likely to degrade in solution.
“Making the GaN more stable is important,” Wilkins adds, “because that makes it more viable for use in biomedical applications, such as implantable sensors.”
The researchers started with polar GaN, composed of alternating layers of gallium and nitrogen. To increase luminescence, they etched the surface of the material with phosphoric acid. At the same time, they added phosphonic groups – organic molecules containing phosphorus – that self-assembled into a monolayer on the surface of the material. This layer further increased luminescence and improved the stability of the GaN by making it less likely to react chemically with its environment.
The paper, “In Situ Chemical Functionalization of Gallium Nitride with Phosphonic Acid Derivatives during Etching,” is published online in the journal Langmuir. Senior author of the paper is Dr. Albena Ivanisevic, an associate professor of materials science and engineering at NC State and associate professor of the joint biomedical engineering program at NC State and the University of North Carolina at Chapel Hill. The paper was co-authored by Dr. Consuelo Arellano, a research associate professor of statistics at NC State; Dr. Tania Paskova, a research professor of electrical and computer engineering at NC State; and Michelle Greenough, an undergraduate at Wagner College.
Abstract of the Paper:
In situ functionalization of polar (c plane) and nonpolar (a plane) gallium nitride (GaN) was performed by adding (3-bromopropyl) phosphonic acid or propyl phosphonic acid to a phosphoric acid etch. The target was to modulate the emission properties and oxide formation of GaN, which was explored through surface characterization with atomic force microscopy, X-ray photoelectron spectroscopy, photoluminescence (PL), inductively coupled plasma–mass spectrometry, and water contact angle. The use of (3-bromopropyl) phosphonic acid and propyl phosphonic acid in phosphoric acid demonstrated lower amounts of gallium oxide formation and greater hydrophobicity for both sample sets, while also improving PL emission of polar GaN samples. In addition to crystal orientation, growth-related factors such as defect density in bulk GaN versus thin GaN films residing on sapphire substrates were investigated as well as their responses to in situ functionalization. Thin nonpolar GaN layers were the most sensitive to etching treatments due in part to higher defect densities (stacking faults and threading dislocations), which accounts for large surface depressions. High-quality GaN (both free-standing bulk polar and bulk nonpolar) demonstrated increased sensitivity to oxide formation. Room-temperature PL stands out as an excellent technique to identify nonradiative recombination as observed in the spectra of heteroepitaxially grown GaN samples. The chemical methods applied to tune optical and physical properties of GaN provide a quantitative framework for future novel chemical and biochemical sensor development.
You can contact Ivanisevic directly at (919) 515-4683 or ivanisevic@ncsu.edu.
More information available at http://news.ncsu.edu/releases/wms-ivanisevic-phosphonic2014/
The research was supported in part by the National Science Foundation under grant EEC 1156762.